The presence of an embryonic opercular flap in amniotes
- PMID: 21632625
- PMCID: PMC3223672
- DOI: 10.1098/rspb.2011.0740
The presence of an embryonic opercular flap in amniotes
Abstract
The operculum is a large flap consisting of several flat bones found on the side of the head of bony fish. During development, the opercular bones form within the second pharyngeal arch, which expands posteriorly and comes to cover the gill-bearing arches. With the evolution of the tetrapods and the assumption of a terrestrial lifestyle, it was believed that the operculum was lost. Here, we demonstrate that an embryonic operculum persists in amniotes and that its early development is homologous with that of teleosts. As in zebrafish, the second pharyngeal arch of the chick embryo grows disproportionately and comes to cover the posterior arches. We show that the developing second pharyngeal arch in both chick and zebrafish embryos express orthologous genes and require shh signalling for caudal expansion. In amniotes, however, the caudal edge of the expanded second arch fuses to the surface of the neck. We have detailed how this process occurs and also demonstrated a requirement for thyroid signalling here. Our results thus demonstrate the persistence of an embryonic opercular flap in amniotes, that its fusion mirrors aspects of amphibian metamorphosis and gives insights into the origin of branchial cleft anomalies in humans.
Figures
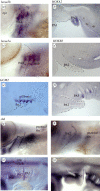
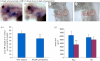
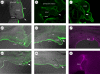
References
-
- Brazeau M. D., Ahlberg P. E. 2006. Tetrapod-like middle ear architecture in a Devonian fish. Nature 439, 318–32110.1038/nature04196 (doi:10.1038/nature04196) - DOI - DOI - PubMed
-
- Daeschler E. B., Shubin N. H., Jenkins F. A., Jr 2006. A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature 440, 757–76310.1038/nature04639 (doi:10.1038/nature04639) - DOI - DOI - PubMed
-
- Kardong K. 1998. Vertebrates: comparative anatomy, function and evolution. New York, NY: McGraw-Hill
-
- Okabe M., Graham A. 2004. The origin of the parathyroid gland. Proc. Natl Acad. Sci. USA 101, 17 716–17 71910.1073/pnas.0406116101 (doi:10.1073/pnas.0406116101) - DOI - DOI - PMC - PubMed
-
- Schoenwolf G. C., Bleyl S. B., Brauer P. R., Francis-West P. H. 2009. Larsen's human embryology. New York, NY: Churchill Livingstone
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

