The 2.3-angstrom structure of porcine circovirus 2
- PMID: 21632760
- PMCID: PMC3147897
- DOI: 10.1128/JVI.00737-11
The 2.3-angstrom structure of porcine circovirus 2
Erratum in
- J Virol. 2011 Nov;85(21):11542
Abstract
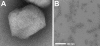
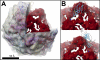
Porcine circovirus 2 (PCV2) is a T=1 nonenveloped icosahedral virus that has had severe impact on the swine industry. Here we report the crystal structure of an N-terminally truncated PCV2 virus-like particle at 2.3-Å resolution, and the cryo-electron microscopy (cryo-EM) image reconstruction of a full-length PCV2 virus-like particle at 9.6-Å resolution. This is the first atomic structure of a circovirus. The crystal structure revealed that the capsid protein fold is a canonical viral jelly roll. The loops connecting the strands of the jelly roll define the limited features of the surface. Sulfate ions interacting with the surface and electrostatic potential calculations strongly suggest a heparan sulfate binding site that allows PCV2 to gain entry into the cell. The crystal structure also allowed previously determined epitopes of the capsid to be visualized. The cryo-EM image reconstruction showed that the location of the N terminus, absent in the crystal structure, is inside the capsid. As the N terminus was previously shown to be antigenic, it may externalize through viral "breathing."
Figures



References
-
- Bothner B., Dong X. F., Bibbs L., Johnson J. E., Siuzdak G. 1998. Evidence of viral capsid dynamics using limited proteolysis and mass spectrometry. J. Biol. Chem. 273:673–676 - PubMed
-
- Brunger A. T., et al. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54:905–921 - PubMed
-
- Cardin A. D., Weintraub H. J. 1989. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9:21–32 - PubMed
-
- Clark E. G. 1997. Post-weaning multisystemic wasting syndrome, p. 499–501 In Proceedings of the 28th Annual Meeting of the American Association of Swine Practitioners, Quebec City, Quebec, Canada: American Association of Swine Practitioners, Perry, IA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

