Regulation of the Dbp5 ATPase cycle in mRNP remodeling at the nuclear pore: a lively new paradigm for DEAD-box proteins
- PMID: 21632821
- PMCID: PMC3110949
- DOI: 10.1101/gad.2062611
Regulation of the Dbp5 ATPase cycle in mRNP remodeling at the nuclear pore: a lively new paradigm for DEAD-box proteins
Abstract
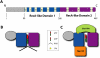
It is commonly assumed that all DEAD-box ATPases function via a shared mechanism, since this is the case for the few proteins characterized thus far. Hodge and colleagues (pp. 1052-1064) and Noble and colleagues (pp. 1065-1077) now describe a novel model for Dbp5's ATPase cycle in mRNA (messenger RNA)/protein complex (mRNP) remodeling during nuclear export. Notably, unlike other DEAD-box proteins, Dbp5 uses a conformational change distinct from ATP hydrolysis for its activity and requires an ADP release factor to reset its ATPase cycle.
Figures

Comment on
-
The Dbp5 cycle at the nuclear pore complex during mRNA export I: dbp5 mutants with defects in RNA binding and ATP hydrolysis define key steps for Nup159 and Gle1.Genes Dev. 2011 May 15;25(10):1052-64. doi: 10.1101/gad.2041611. Genes Dev. 2011. PMID: 21576265 Free PMC article.
-
The Dbp5 cycle at the nuclear pore complex during mRNA export II: nucleotide cycling and mRNP remodeling by Dbp5 are controlled by Nup159 and Gle1.Genes Dev. 2011 May 15;25(10):1065-77. doi: 10.1101/gad.2040611. Genes Dev. 2011. PMID: 21576266 Free PMC article.
References
-
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. 2007. The molecular architecture of the nuclear pore complex. Nature 450: 695–701 - PubMed
-
- Andersen CB, Ballut L, Johansen JS, Chamieh H, Nielsen KH, Oliveira CL, Pedersen JS, Seraphin B, Le Hir H, Andersen GR 2006. Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science 313: 1968–1972 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
