Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice
- PMID: 21632826
- PMCID: PMC3110957
- DOI: 10.1101/gad.2029411
Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice
Abstract
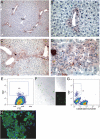
The molecular identification of adult hepatic stem/progenitor cells has been hampered by the lack of truly specific markers. To isolate putative adult liver progenitor cells, we used cell surface-marking antibodies, including MIC1-1C3, to isolate subpopulations of liver cells from normal adult mice or those undergoing an oval cell response and tested their capacity to form bilineage colonies in vitro. Robust clonogenic activity was found to be restricted to a subset of biliary duct cells antigenically defined as CD45(-)/CD11b(-)/CD31(-)/MIC1-1C3(+)/CD133(+)/CD26(-), at a frequency of one of 34 or one of 25 in normal or oval cell injury livers, respectively. Gene expression analyses revealed that Sox9 was expressed exclusively in this subpopulation of normal liver cells and was highly enriched relative to other cell fractions in injured livers. In vivo lineage tracing using Sox9creER(T2)-R26R(YFP) mice revealed that the cells that proliferate during progenitor-driven liver regeneration are progeny of Sox9-expressing precursors. A comprehensive array-based comparison of gene expression in progenitor-enriched and progenitor-depleted cells from both normal and DDC (3,5-diethoxycarbonyl-1,4-dihydrocollidine or diethyl1,4-dihydro-2,4,6-trimethyl-3,5-pyridinedicarboxylate)-treated livers revealed new potential regulators of liver progenitors.
Figures




References
-
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. 2010. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36 - PubMed
-
- Clayton E, Forbes SJ 2009. The isolation and in vitro expansion of hepatic Sca-1 progenitor cells. Biochem Biophys Res Commun 381: 549–553 - PubMed
-
- Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA 2003. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4: 3 doi: 10.1186/gb-2003-4-5-p3 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
