Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response
- PMID: 21632851
- PMCID: PMC3657705
- DOI: 10.1158/1078-0432.CCR-11-0815
Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response
Abstract
Purpose: Abiraterone is an oral inhibitor of CYP17, which is essential for androgen biosynthesis. This multicenter study assessed its efficacy in patients with castration-resistant prostate cancer (CRPC), without prior chemotherapy or CYP17-targeted therapy, and frequency of bone scans discordant with prostate-specific antigen (PSA) and clinical response.
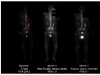
Experimental design: Thirty-three patients received abiraterone acetate 1,000 mg daily with prednisone 5 mg twice daily in continuous 28-day cycles. Patients were evaluated monthly for efficacy and safety. Bone scan flare was defined as the combination, after 3 months of therapy, of an interpreting radiologist's report indicating "disease progression" in context of a 50% or more decline in PSA level, with scan improvement or stability 3 months later.
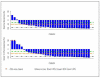
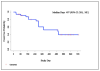
Results: A 50% or more decline in PSA level at week 12 was confirmed in 22 of 33 (67%) patients. Declines in PSA level of 50% or more were seen in 26 of 33 (79%) patients. Undetectable PSA levels (≤0.1 ng/mL) occurred in 2 patients. Median time on therapy and time to PSA progression were 63 weeks and 16.3 months, respectively. Twenty-three patients were evaluable for bone scan flare. Progression was indicated in radiologist's report in 12 of 23 (52%), and 11 of 12 subsequently showed improvement or stability. As prospectively defined, bone scan flare was observed in 11 of 23 (48%) evaluable patients or 11 of 33 (33%) enrolled patients. Adverse events were typically grade 1/2 and consistent with prior published abiraterone reports.
Conclusion: Clinical responses to abiraterone plus prednisone were frequent and durable in men with metastatic CRPC. Further investigation is needed to clarify the confounding effect of bone scan flare on patient management and interpretation of results. Clin Cancer Res; 17(14); 4854-61. ©2011 AACR.
Figures


References
-
- Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–99. - PubMed
-
- Mostaghel EA, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67(10):5033–41. - PubMed
-
- Small E, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

