Cardiac-specific mindin overexpression attenuates cardiac hypertrophy via blocking AKT/GSK3β and TGF-β1-Smad signalling
- PMID: 21632881
- PMCID: PMC3657538
- DOI: 10.1093/cvr/cvr159
Cardiac-specific mindin overexpression attenuates cardiac hypertrophy via blocking AKT/GSK3β and TGF-β1-Smad signalling
Abstract
Aims: Mindin is a secreted extracellular matrix protein, an integrin ligand, and an angiogenesis inhibitor, other examples of which are all key players in the progression of cardiac hypertrophy. However, its function during cardiac hypertrophy remains unclear. This study was aimed to identify the effect of mindin on cardiac hypertrophy and the underlying mechanisms.
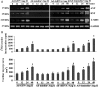
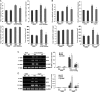
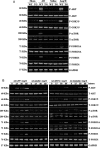
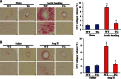
Methods and results: A significant down-regulation of mindin expression was observed in human failing hearts. To further investigate the role of mindin in cardiac hypertrophy, we used cultured neonatal rat cardiomyocytes with gain and loss of mindin function and cardiac-specific Mindin-overexpressing transgenic (TG) mice. In cultured cardiomyocytes, mindin negatively regulated angiotensin II (Ang II)-mediated hypertrophic growth, as detected by [(3)H]-Leucine incorporation, cardiac myocyte area, and hypertrophic marker protein levels. Cardiac hypertrophy in vivo was produced by aortic banding (AB) or Ang II infusion in TG mice and their wild-type controls. The extent of cardiac hypertrophy was evaluated by echocardiography as well as by pathological and molecular analyses of heart samples. Mindin overexpression in the heart markedly attenuated cardiac hypertrophy, fibrosis, and left ventricular dysfunction in mice in response to AB or Ang II. Further analysis of the signalling events in vitro and in vivo indicated that these beneficial effects of mindin were associated with the interruption of AKT/glycogen synthase kinase 3β (GSK3β) and transforming growth factor (TGF)-β1-Smad signalling.
Conclusion: The present study demonstrates for the first time that mindin serves as a novel mediator that protects against cardiac hypertrophy and the transition to heart failure by blocking AKT/GSK3β and TGF-β1-Smad signalling.
Figures


References
-
- Swynghedauw B, Delcayre C, Samuel JL, Mebazaa A, Cohen-Solal A. Molecular mechanisms in evolutionary cardiology failure. Ann N Y Acad Sci. 2010;1188:58–67. doi:10.1111/j.1749-6632.2009.05084.x. - DOI - PubMed
-
- Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi:10.1161/CIRCULATIONAHA.107.702795. - DOI - PubMed
-
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. - PubMed
-
- Jane-Lise S, Corda S, Chassagne C, Rappaport L. The extracellular matrix and the cytoskeleton in heart hypertrophy and failure. Heart Fail Rev. 2000;5:239–250. doi:10.1023/A:1009857403356. - DOI - PubMed
-
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi:10.1126/science.285.5430.1028. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

