Sparse but highly efficient Kv3 outpace BKCa channels in action potential repolarization at hippocampal mossy fiber boutons
- PMID: 21632922
- PMCID: PMC6622861
- DOI: 10.1523/JNEUROSCI.0972-11.2011
Sparse but highly efficient Kv3 outpace BKCa channels in action potential repolarization at hippocampal mossy fiber boutons
Abstract
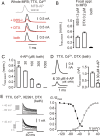
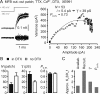
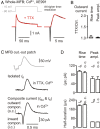
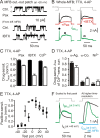
Presynaptic elements of axons, in which action potentials (APs) cause release of neurotransmitter, are sites of high densities and complex interactions of proteins. We report that the presence of K(v)3 channels in addition to K(v)1 at glutamatergic mossy fiber boutons (MFBs) in rat hippocampal slices considerably limits the number of fast, voltage-activated potassium channels necessary to achieve basal presynaptic AP repolarization. The ∼ 10-fold higher repolarization efficacy per K(v)3 channel compared with presynaptic K(v)1 results from a higher steady-state availability at rest, a better recruitment by the presynaptic AP as a result of faster activation kinetics, and a larger single-channel conductance. Large-conductance calcium- and voltage-activated potassium channels (BK(Ca)) at MFBs give rise to a fast activating/fast inactivating and a slowly activating/sustained K(+) current component during long depolarizations. However, BK(Ca) contribute to MFB-AP repolarization only after presynaptic K(v)3 have been disabled. The calcium chelators EGTA and BAPTA are equally effective in preventing BK(Ca) activation, suggesting that BK(Ca) are not organized in nanodomain complexes with presynaptic voltage-gated calcium channels. Thus, the functional properties of K(v)3 channels at MFBs are tuned to both promote brevity of presynaptic APs limiting glutamate release and at the same time keep surface protein density of potassium channels low. Presynaptic BK(Ca) channels are restricted to limit additional increases of the AP half-duration in case of K(v)3 hypofunction, because rapid membrane repolarization by K(v)3 combined with distant calcium sources prevent BK(Ca) activation during basal APs.
Figures


References
-
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. - PubMed
-
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. - PubMed
-
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. - PubMed
-
- Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci. 2003;6:258–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
