Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa
- PMID: 21632926
- PMCID: PMC3112354
- DOI: 10.1523/JNEUROSCI.6671-10.2011
Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa
Abstract
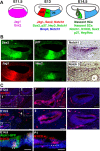
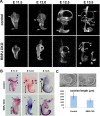
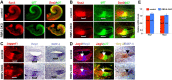
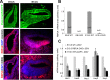
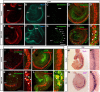
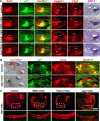
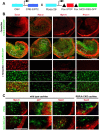
The mammalian organ of Corti consists of a highly organized array of hair cells and supporting cells that originate from a common population of prosensory progenitors. Proper differentiation of this complex cellular mosaic requires lateral inhibition mediated by Notch signaling. Several studies have implicated Notch signaling in the earlier induction of the prosensory domain that lies along the length of the cochlear duct, and which forms before the onset of hair cell and supporting cell differentiation. To investigate the role of Notch signaling in prosensory domain formation, we conditionally inactivated the transcriptional mediator of canonical Notch signaling, RBPjκ, throughout the inner ear. Although RBPjκ mutants have severe vestibular defects and a shortened cochlear duct, markers of the prosensory domain appear at the normal time and location in the cochlea of RBPjκ mutants. Despite the lack of RBPjκ, hair cell and supporting cell markers also appear at appropriate times in the cochlea, suggesting that RBPjκ is dispensable for differentiation of the cochlear sensory epithelium. However, we also observed that differentiating hair cells and supporting cells rapidly die in RBPjκ mutants, suggesting a requirement of RBPjκ for cell survival in this tissue. Finally, in contrast to the chick basilar papilla, ectopic activation of Notch signaling did not induce ectopic sensory patches in nonsensory regions of the cochlea. Our results indicate that canonical Notch signaling is not necessary for prosensory specification in the mouse cochlea, suggesting that other signaling pathways may specify this highly derived sensory organ.
Figures
References
-
- Bok J, Chang W, Wu DK. Patterning and morphogenesis of the vertebrate inner ear. Int J Dev Biol. 2007;51:521–533. - PubMed
-
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. - PubMed
-
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
