A silent synapse-based mechanism for cocaine-induced locomotor sensitization
- PMID: 21632938
- PMCID: PMC3286116
- DOI: 10.1523/JNEUROSCI.0016-11.2011
A silent synapse-based mechanism for cocaine-induced locomotor sensitization
Abstract
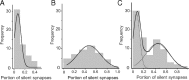
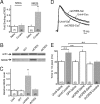
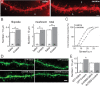
Locomotor sensitization is a common and robust behavioral alteration in rodents whereby following exposure to abused drugs such as cocaine, the animal becomes significantly more hyperactive in response to an acute drug challenge. Here, we further analyzed the role of cocaine-induced silent synapses in the nucleus accumbens (NAc) shell and their contribution to the development of locomotor sensitization. Using a combination of viral vector-mediated genetic manipulations, biochemistry, and electrophysiology in a locomotor sensitization paradigm with repeated, daily, noncontingent cocaine (15 mg/kg) injections, we show that dominant-negative cAMP-element binding protein (CREB) prevents cocaine-induced generation of silent synapses of young (30 d old) rats, whereas constitutively active CREB is sufficient to increase the number of NR2B-containing NMDA receptors (NMDARs) at synapses and to generate silent synapses. We further show that occupancy of CREB at the NR2B promoter increases and is causally related to the increase in synaptic NR2B levels. Blockade of NR2B-containing NMDARs by administration of the NR2B-selective antagonist Ro256981 directly into the NAc, under conditions that inhibit cocaine-induced silent synapses, prevents the development of cocaine-elicited locomotor sensitization. Our data are consistent with a cellular cascade whereby cocaine-induced activation of CREB promotes CREB-dependent transcription of NR2B and synaptic incorporation of NR2B-containing NMDARs, which generates new silent synapses within the NAc. We propose that cocaine-induced activation of CREB and generation of new silent synapses may serve as key cellular events mediating cocaine-induced locomotor sensitization. These findings provide a novel cellular mechanism that may contribute to cocaine-induced behavioral alterations.
Figures


References
-
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. - PubMed
-
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. - PubMed
-
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol. 1992;68:945–960. - PubMed
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
