Deletion of astroglial Dicer causes non-cell-autonomous neuronal dysfunction and degeneration
- PMID: 21632951
- PMCID: PMC3500097
- DOI: 10.1523/JNEUROSCI.0567-11.2011
Deletion of astroglial Dicer causes non-cell-autonomous neuronal dysfunction and degeneration
Abstract
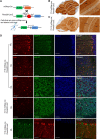
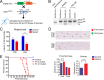
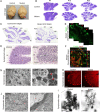
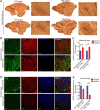
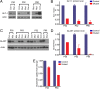
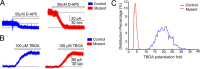
The endoribonuclease, Dicer, is indispensable for generating the majority of mature microRNAs (miRNAs), which are posttranscriptional regulators of gene expression involved in a wide range of developmental and pathological processes in the mammalian CNS. Although functions of Dicer-dependent miRNA pathways in neurons and oligodendrocytes have been extensively investigated, little is known about the role of Dicer in astrocytes. Here, we report the effect of Cre-loxP-mediated conditional deletion of Dicer selectively from postnatal astroglia on brain development. Dicer-deficient mice exhibited normal motor development and neurological morphology before postnatal week 5. Thereafter, mutant mice invariably developed a rapidly fulminant neurological decline characterized by ataxia, severe progressive cerebellar degeneration, seizures, uncontrollable movements, and premature death by postnatal week 9-10. Integrated transcription profiling, histological, and functional analyses of cerebella showed that deletion of Dicer in cerebellar astrocytes altered the transcriptome of astrocytes to be more similar to an immature or reactive-like state before the onset of neurological symptoms or morphological changes. As a result, critical and mature astrocytic functions including glutamate uptake and antioxidant pathways were substantially impaired, leading to massive apoptosis of cerebellar granule cells and degeneration of Purkinje cells. Collectively, our study demonstrates the critical involvement of Dicer in normal astrocyte maturation and maintenance. Our findings also reveal non-cell-autonomous roles of astrocytic Dicer-dependent pathways in regulating proper neuronal functions and implicate that loss of or dysregulation of astrocytic Dicer-dependent pathways may be involved in neurodegeneration and other neurological disorders.
Figures

References
-
- Apelt J, Schliebs R. Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894:21–30. - PubMed
-
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
