Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis
- PMID: 21632984
- PMCID: PMC3719858
- DOI: 10.1126/scitranslmed.3002231
Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis
Abstract
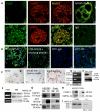
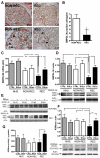
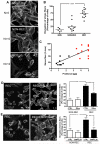
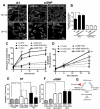
Focal segmental glomerulosclerosis (FSGS) is a glomerular disease characterized by proteinuria, progression to end-stage renal disease, and recurrence of proteinuria after kidney transplantation in about one-third of patients. It has been suggested that rituximab might treat recurrent FSGS through an unknown mechanism. Rituximab not only recognizes CD20 on B lymphocytes, but might also bind sphingomyelin phosphodiesterase acid-like 3b (SMPDL-3b) protein and regulate acid sphingomyelinase (ASMase) activity. We hypothesized that rituximab prevents recurrent FSGS and preserves podocyte SMPDL-3b expression. We studied 41 patients at high risk for recurrent FSGS, 27 of whom were treated with rituximab at time of kidney transplant. SMPDL-3b protein, ASMase activity, and cytoskeleton remodeling were studied in cultured normal human podocytes that had been exposed to patient sera with or without rituximab. Rituximab treatment was associated with lower incidence of posttransplant proteinuria and stabilization of glomerular filtration rate. The number of SMPDL-3b(+) podocytes in postreperfusion biopsies was reduced in patients who developed recurrent FSGS. Rituximab partially prevented SMPDL-3b and ASMase down-regulation that was observed in podocytes treated with the sera of patients with recurrent FSGS. Overexpression of SMPDL-3b or treatment with rituximab was able to prevent disruption of the actin cytoskeleton and podocyte apoptosis induced by patient sera. This effect was diminished in cultured podocytes where SMPDL-3b was silenced. Our study suggests that treatment of high-risk patients with rituximab at time of kidney transplant might prevent recurrent FSGS by modulating podocyte function in an SMPDL-3b-dependent manner.
Figures
Comment in
-
Rituximab's new therapeutic target: the podocyte actin cytoskeleton.Sci Transl Med. 2011 Jun 1;3(85):85ps21. doi: 10.1126/scitranslmed.3002429. Sci Transl Med. 2011. PMID: 21632983
References
-
- Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815–825. - PubMed
-
- Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. - PubMed
-
- Baum MA. Outcomes after renal transplantation for FSGS in children. Pediatr Transplant. 2004;8:329–333. - PubMed
-
- Senggutuvan P, Cameron JS, Hartley RB, Rigden S, Chantler C, Haycock G, Williams DG, Ogg C, Koffman G. Recurrence of focal segmental glomerulosclerosis in transplanted kidneys: analysis of incidence and risk factors in 59 allografts. Pediatr Nephrol. 1990;4:21–28. - PubMed
-
- Hubsch H, Montane B, Abitbol C, Chandar J, Shariatmadar S, Ciancio G, Burke G, Miller J, Strauss J, Zilleruelo G. Recurrent focal glomerulosclerosis in pediatric renal allografts: the Miami experience. Pediatr Nephrol. 2005;20:210–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EB008009/EB/NIBIB NIH HHS/United States
- DK73495/DK/NIDDK NIH HHS/United States
- 5 R01 EB008009-03/EB/NIBIB NIH HHS/United States
- R43 DK083832-11/DK/NIDDK NIH HHS/United States
- U01 DK070460/DK/NIDDK NIH HHS/United States
- DK82636/DK/NIDDK NIH HHS/United States
- R01 DK073495/DK/NIDDK NIH HHS/United States
- DK089304/DK/NIDDK NIH HHS/United States
- R01 DK070011/DK/NIDDK NIH HHS/United States
- R43 DK083832/DK/NIDDK NIH HHS/United States
- 1U01 DK70460-06/DK/NIDDK NIH HHS/United States
- R01 DK090316/DK/NIDDK NIH HHS/United States
- K08 DK082636/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

