MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines
- PMID: 21632986
- PMCID: PMC3501657
- DOI: 10.1126/scitranslmed.3002336
MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines
Abstract
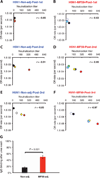
Oil-in-water adjuvants have been shown to improve immune responses against pandemic influenza vaccines as well as reduce the effective vaccine dose, increasing the number of doses available to meet global vaccine demand. Here, we use genome fragment phage display libraries and surface plasmon resonance to elucidate the effects of MF59 on the quantity, diversity, specificity, and affinity maturation of human antibody responses to the swine-origin H1N1 vaccine in different age groups. In adults and children, MF59 selectively enhanced antibody responses to the hemagglutinin 1 (HA1) globular head relative to the more conserved HA2 domain in terms of increased antibody titers as well as a more diverse antibody epitope repertoire. Antibody affinity, as inferred by greatly diminished (≥10-fold) off-rate constants, was significantly increased in toddlers and children who received the MF59-adjuvanted vaccine. Moreover, MF59 also improved antibody affinity maturation after each sequential vaccination against avian H5N1 in adults. For both pandemic influenza vaccines, there was a close correlation between serum antibody affinity and virus-neutralizing capacity. Thus, MF59 quantitatively and qualitatively enhances functional antibody responses to HA-based vaccines by improving both epitope breadth and binding affinity, demonstrating the added value of such adjuvants for influenza vaccines.
Figures






Comment in
-
Antibody repertoire: embracing diversity.Sci Transl Med. 2011 Jul 27;3(93):93ps32. doi: 10.1126/scitranslmed.3002694. Sci Transl Med. 2011. PMID: 21795586
References
-
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 2006;354:1343–1351. - PubMed
-
- Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, Noah DL, He F, Hill H. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J. Infect. Dis. 2008;197:667–675. - PubMed
-
- Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, Zhang XF, Pan HX, Meng FY, Hu YM, Liu WD, Li CG, Li W, Zhang X, Hu JM, Peng WB, Yang BP, Xi P, Wang HQ, Zheng JS. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 2009;361:2414–2423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

