C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration
- PMID: 21633121
- PMCID: PMC4916773
- DOI: 10.1136/bjo.2010.199216
C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration
Abstract
Background: There is increasing evidence that inflammation and immune-mediated processes (complement activation) play an important role in age-related macular degeneration (AMD) pathogenesis. A genetic variation in the gene encoding complement factor H (CFH) and plasma levels of C-reactive protein (CRP), a systemic marker of subclinical inflammation, have consistently been shown to be associated with an increased risk for AMD. In the present study, we examined the immunolocalisation of CRP and CFH in aged control human donor eyes (n=10; mean age 79 years) and eyes with AMD (n=18; mean age 83 years).
Methods: Alkaline phosphatase immunohistochemistry was performed using polyclonal antibodies against CRP and CFH on cryopreserved tissue sections from disc/macular blocks. Three independent masked observers scored the reaction product (0-8).
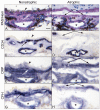
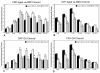
Results: In aged control eyes, the retinal pigment epithelium/Bruch's membrane/choriocapillaris (RPE/BrM/CC) complex including intercapillary septa (ICS) had the most prominent immunostaining for CRP and CFH. CRP was significantly higher than controls in BrM/CC/ICS and choroidal stroma in early and wet AMD eyes (p<0.05). In contrast, CFH was significantly lower in BrM/CC/ICS complex of AMD choroids than in controls (p<0.05). Interestingly, CRP and CFH were significantly reduced in BrM/CC/ICS complex in atrophic area of macula in geographical atrophy (p<0.05). Drusen and basal laminar deposits were intensely positive for CRP and CFH.
Conclusion: These immunohistochemical findings show that changes in distribution and relative levels of CRP and CFH were evident in early and late AMD eyes. This suggests that high levels of CRP and insufficient CFH at the retina/choroid interface may lead to uncontrolled complement activation with associated cell and tissue damage. This study supports the hypothesis that inflammation and immune-mediated mechanisms are involved in the pathogenesis of AMD.
Figures



References
-
- Ambati J, Ambati BK, Yoo SH, et al. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. - PubMed
-
- Johnson LV, Ozaki S, Staples MK, et al. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449. - PubMed
-
- Johnson LV, Leitner WP, Staples MK, et al. Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–896. - PubMed
-
- Hageman GS, Mullins RF, Russell SR, et al. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13:477–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
