The three R's of lung health and disease: repair, remodeling, and regeneration
- PMID: 21633173
- PMCID: PMC3104764
- DOI: 10.1172/JCI45961
The three R's of lung health and disease: repair, remodeling, and regeneration
Abstract
All tissues and organs can be classified according to their ability to repair and regenerate during adult homeostasis and after injury. Some exhibit a high rate of constant cell turnover, while others, such as the lung, exhibit only low-level cell regeneration during normal adult homeostasis but have the ability to rapidly regenerate new cells after injury. Lung regeneration likely involves both activation of progenitor cells as well as cell replacement through proliferation of remaining undamaged cells. The pathways and factors that control this process and its role in disease are only now being explored. In this Review, we will discuss the connection between pathways required for lung development and how the lung responds to injury and disease, with a particular emphasis on recent studies describing the role for the epithelium in repair and regeneration.
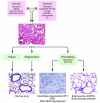
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- R01 ES015857/ES/NIEHS NIH HHS/United States
- R01 HL071589/HL/NHLBI NIH HHS/United States
- R01 HL087825/HL/NHLBI NIH HHS/United States
- P01 HL019737/HL/NHLBI NIH HHS/United States
- HL19737/HL/NHLBI NIH HHS/United States
- R01 HL064632/HL/NHLBI NIH HHS/United States
- HL071589/HL/NHLBI NIH HHS/United States
- HL087825/HL/NHLBI NIH HHS/United States
- HL100405/HL/NHLBI NIH HHS/United States
- P30 ES015857/ES/NIEHS NIH HHS/United States
- U01 HL100405/HL/NHLBI NIH HHS/United States
- HL087177/HL/NHLBI NIH HHS/United States
- R01 HL087177/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

