Dynamics of insulin secretion and the clinical implications for obesity and diabetes
- PMID: 21633180
- PMCID: PMC3104758
- DOI: 10.1172/JCI45680
Dynamics of insulin secretion and the clinical implications for obesity and diabetes
Abstract
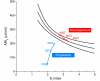
Insulin secretion is a highly dynamic process regulated by various factors including nutrients, hormones, and neuronal inputs. The dynamics of insulin secretion can be studied at different levels: the single β cell, pancreatic islet, whole pancreas, and the intact organism. Studies have begun to analyze cellular and molecular mechanisms underlying dynamics of insulin secretion. This review focuses on our current understanding of the dynamics of insulin secretion in vitro and in vivo and discusses their clinical relevance.
Figures

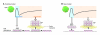

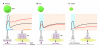
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

