HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine
- PMID: 21633285
- PMCID: PMC4547386
- DOI: 10.1097/QAD.0b013e3283492180
HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine
Abstract
Objective: To describe the prevalence of HIV-1 drug resistance mutations at the time of treatment initiation in a large cohort of HIV-infected children previously exposed to single-dose nevirapine (sdNVP) for prevention of transmission.
Design: Drug resistance mutations were measured pretreatment in 255 infants and young children under 2 years of age in South Africa exposed to sdNVP and initiating ritonavir-boosted lopinavir-based therapy. Those who achieved viral suppression were randomized to either continue the primary regimen or to switch to a nevirapine-based regimen. Pretreatment samples were tested using population sequencing and real time allele-specific PCR (AS-PCR) to detect Y181C and K103N minority variants. Those with confirmed viremia more than 1000 copies/ml by 52 weeks postrandomization in the switch group were defined as having viral failure.
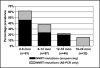
Results: Nonnucleoside reverse transcriptase inhibitor (NNRTI) mutations, predominantly Y181C, were detected by either method in 62% of infants less than 6 months of age, in 39% of children 6-12 months of age, 22% 12-18 months, and 16% 18-24 months (P = <0.0001). NNRTI mutations detected by genotyping, but not K103N or Y181C mutations detected only by AS-PCR, were associated with viral failure in the switch group.
Conclusion: The prevalence of mutations known to compromise primary NNRTI-based therapy is high in sdNVP-exposed children, supporting current guidelines recommending use of protease inhibitor-based regimens for young children. Standard genotyping is adequate to identify children who could benefit from switching to NNRTI-based therapy.
Figures


References
-
- Fowler MG, Lampe MA, Jamieson DJ, Kourtis AP, Rogers MF. Reducing the risk of mother-to-child human immunodeficiency virus transmission: past successes, current progress and challenges, and future directions. Am J Obstet Gynecol. 2007;197:S3–9. - PubMed
-
- Eshleman SH, Mracna M, Guay LA, Deseyve M, Cunningham S, Mirochnick M, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012). Aids. 2001;15:1951–1957. - PubMed
-
- Johnson JA, Li JF, Morris L, Martinson N, Gray G, McIntyre J, Heneine W. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192:16–23. - PubMed
-
- Flys TS, Mwatha A, Guay LA, Nakabiito C, Donnell D, Musoke P, et al. Detection of K103N in Ugandan women after repeated exposure to single dose nevirapine. Aids. 2007;21:2077–2082. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

