The N-end rule pathway and regulation by proteolysis
- PMID: 21633985
- PMCID: PMC3189519
- DOI: 10.1002/pro.666
The N-end rule pathway and regulation by proteolysis
Abstract
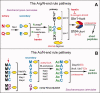
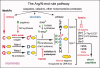
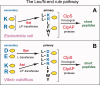
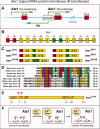
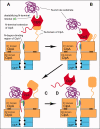
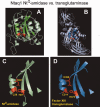
The N-end rule relates the regulation of the in vivo half-life of a protein to the identity of its N-terminal residue. Degradation signals (degrons) that are targeted by the N-end rule pathway include a set called N-degrons. The main determinant of an N-degron is a destabilizing N-terminal residue of a protein. In eukaryotes, the N-end rule pathway is a part of the ubiquitin system and consists of two branches, the Ac/N-end rule and the Arg/N-end rule pathways. The Ac/N-end rule pathway targets proteins containing N(α) -terminally acetylated (Nt-acetylated) residues. The Arg/N-end rule pathway recognizes unacetylated N-terminal residues and involves N-terminal arginylation. Together, these branches target for degradation a majority of cellular proteins. For example, more than 80% of human proteins are cotranslationally Nt-acetylated. Thus most proteins harbor a specific degradation signal, termed (Ac)N-degron, from the moment of their birth. Specific N-end rule pathways are also present in prokaryotes and in mitochondria. Enzymes that produce N-degrons include methionine-aminopeptidases, caspases, calpains, Nt-acetylases, Nt-amidases, arginyl-transferases and leucyl-transferases. Regulated degradation of specific proteins by the N-end rule pathway mediates a legion of physiological functions, including the sensing of heme, oxygen, and nitric oxide; selective elimination of misfolded proteins; the regulation of DNA repair, segregation and condensation; the signaling by G proteins; the regulation of peptide import, fat metabolism, viral and bacterial infections, apoptosis, meiosis, spermatogenesis, neurogenesis, and cardiovascular development; and the functioning of adult organs, including the pancreas and the brain. Discovered 25 years ago, this pathway continues to be a fount of biological insights.
Figures









References
-
- Varshavsky A. Spalog and sequelog: neutral terms for spatial and sequence similarity. Curr Biol. 2004;14:R181–R183. - PubMed
-
- Varshavsky A. Naming a targeting signal. Cell. 1991;64:13–15. - PubMed
-
- Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

