T regulatory cells participate in the control of germinal centre reactions
- PMID: 21635248
- PMCID: PMC3143357
- DOI: 10.1111/j.1365-2567.2011.03456.x
T regulatory cells participate in the control of germinal centre reactions
Abstract
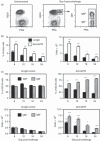
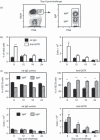
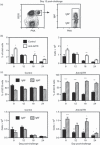
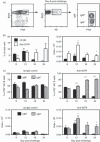
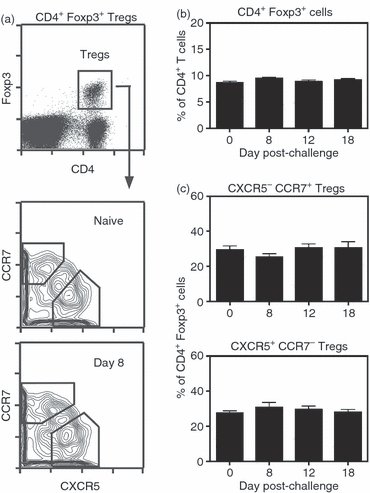
Germinal centre (GC) reactions are central features of T-cell-driven B-cell responses, and the site where antibody-producing cells and memory B cells are generated. Within GCs, a range of complex cellular and molecular events occur which are critical for the generation of high affinity antibodies. These processes require exquisite regulation not only to ensure the production of desired antibodies, but to minimize unwanted autoreactive or low affinity antibodies. To assess whether T regulatory (Treg) cells participate in the control of GC responses, immunized mice were treated with an anti-glucocorticoid-induced tumour necrosis factor receptor-related protein (GITR) monoclonal antibody (mAb) to disrupt Treg-cell activity. In anti-GITR-treated mice, the GC B-cell pool was significantly larger compared with control-treated animals, with switched GC B cells composing an abnormally high proportion of the response. Dysregulated GCs were also observed regardless of strain, T helper type 1 or 2 polarizing antigens, and were also seen after anti-CD25 mAb treatment. Within the spleens of immunized mice, CXCR5(+) and CCR7(-) Treg cells were documented by flow cytometry and Foxp3(+) cells were found within GCs using immunohistology. Final studies demonstrated administration of either anti-transforming growth factor-β or anti-interleukin-10 receptor blocking mAb to likewise result in dysregulated GCs, suggesting that generation of inducible Treg cells is important in controlling the GC response. Taken together, these findings indicate that Treg cells contribute to the overall size and quality of the humoral response by controlling homeostasis within GCs.
© 2011 The Authors. Immunology © 2011 Blackwell Publishing Ltd.
Figures

References
-
- Wolniak KL, Shinall SM, Waldschmidt TJ. The germinal center response. Crit Rev Immunol. 2004;24:39–65. - PubMed
-
- Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. - PubMed
-
- Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–8. - PubMed
-
- Shinall SM, Gonzalez-Fernandez M, Noelle RJ, Waldschmidt TJ. Identification of murine germinal center B cell subsets defined by the expression of surface isotypes and differentiation antigens. J Immunol. 2000;164:5729–38. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

