A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome
- PMID: 21635753
- PMCID: PMC3219001
- DOI: 10.1186/cc10249
A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome
Abstract
Introduction: Tidal volume and plateau pressure minimisation are the standard components of a protective lung ventilation strategy for patients with acute respiratory distress syndrome (ARDS). Open lung strategies, including higher positive end-expiratory pressure (PEEP) and recruitment manoeuvres to date have not proven efficacious. This study examines the effectiveness and safety of a novel open lung strategy, which includes permissive hypercapnia, staircase recruitment manoeuvres (SRM) and low airway pressure with PEEP titration.
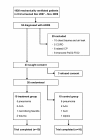
Method: Twenty ARDS patients were randomised to treatment or ARDSnet control ventilation strategies. The treatment group received SRM with decremental PEEP titration and targeted plateau pressure < 30 cm H2O. Gas exchange and lung compliance were measured daily for 7 days and plasma cytokines in the first 24 hours and on days 1, 3, 5 and 7 (mean ± SE). Duration of ventilation, ICU stay and hospital stay (median and interquartile range) and hospital survival were determined.
Results: There were significant overall differences between groups when considering plasma IL-8 and TNF-α. For plasma IL-8, the control group was 41% higher than the treatment group over the seven-day period (ratio 1.41 (1.11 to 1.79), P = 0.01), while for TNF-α the control group was 20% higher over the seven-day period (ratio 1.20 (1.01 to 1.42) P = 0.05). PaO2/FIO2 (204 ± 9 versus 165 ± 9 mmHg, P = 0.005) and static lung compliance (49.1 ± 2.9 versus 33.7 ± 2.7 mls/cm H2O, P < 0.001) were higher in the treatment group than the control group over seven days. There was no difference in duration of ventilation (180 (87 to 298) versus 341 (131 to 351) hrs, P = 0.13), duration of ICU stay (9.9 (5.6 to 14.8) versus 16.0 (8.1 to 19.3) days, P = 0.19) and duration of hospital stay (17.9 (13.7 to 34.5) versus 24.7 (20.5 to 39.8) days, P = 0.16) between the treatment and control groups.
Conclusions: This open lung strategy was associated with greater amelioration in some systemic cytokines, improved oxygenation and lung compliance over seven days. A larger trial powered to examine clinically-meaningful outcomes is warranted.
Trial registration: ACTRN12607000465459.
Figures



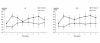
References
-
- Brower RG, Rubenfeld GD. Lung-protective ventilation strategies in acute lung injury. Crit Care Med. 2003;31(4 Suppl):S312–316. - PubMed
-
- Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino G, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. New Engl J Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. - DOI - PubMed
-
- Brower RG, Matthay M, Schoenfeld D. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials. Am J Respir Crit Care Med. 2002;166(11):1515–1517. - PubMed
-
- Petrucci N, Iacovelli W. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2007. p. CD003844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

