Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers
- PMID: 21636129
- PMCID: PMC4163047
- DOI: 10.1016/j.biomaterials.2011.05.006
Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers
Abstract
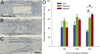
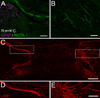
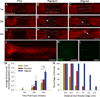
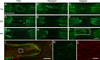
Following spinal cord injury, axons fail to regenerate without exogenous intervention. In this study we report that aligned microfiber-based grafts foster robust regeneration of vascularized CNS tissue. Film, random, and aligned microfiber-based conduits were grafted into a 3 mm thoracic rat spinal cord gap created by complete transection. Over the course of 4 weeks, microtopography presented by aligned or random poly-L-lactic acid microfibers facilitated infiltration of host tissue, and the initial 3 mm gap was closed by endogenous cell populations. This bulk tissue response was composed of regenerating axons accompanied by morphologically aligned astrocytes. Aligned fibers promoted long distance (2055 ± 150 μm), rostrocaudal axonal regeneration, significantly greater than random fiber (1162 ± 87 μm) and film (413 ± 199 μm) controls. Retrograde tracing indicated that regenerating axons originated from propriospinal neurons of the rostral spinal cord, and supraspinal neurons of the reticular formation, red nucleus, raphe and vestibular nuclei. Our findings outline a form of regeneration within the central nervous system that holds important implications for regeneration biology.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





References
-
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. - PubMed
-
- Ylera B, Ertürk A, Hellal F, Nadrigny F, Hurtado A, Tahirovic S, et al. Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr Biol. 2009;19:930–936. - PubMed
-
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

