Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone
- PMID: 21636295
- PMCID: PMC3280079
- DOI: 10.1016/j.immuni.2011.03.024
Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone
Abstract
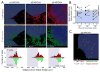
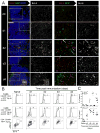
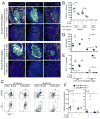
We identify the interfollicular (IF) zone as the site where germinal center B cell and T follicular helper (Tfh) cell differentiation initiates. For the first 2 days postimmunization, antigen-specific T and B cells remained confined within the IF zone, formed long-lived interactions, and upregulated the transcriptional repressor Bcl6. T cells also acquired the Tfh cell markers CXCR5, PD-1, and GL7. Responding B and T cells migrated to the follicle interior directly from the IF zone, T cell immigration preceding B cells by 1 day. Notably, in the absence of cognate B cells, Tfh cells still formed and migrated to the follicle. However, without such B cells, PD-1, ICOS, and GL7 were no longer expressed on follicular Bcl6(hi) T cells that nevertheless persisted in the follicle. Thus, Ag-specific B cells are required for the maintenance of the PD-1(hi)ICOS(hi)GL7(hi) Tfh cell phenotype within the follicle, but not for their initial differentiation in the IF zone.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




Comment in
-
A fine romance: T follicular helper cells and B cells.Immunity. 2011 Jun 24;34(6):827-9. doi: 10.1016/j.immuni.2011.06.007. Immunity. 2011. PMID: 21703537
References
-
- Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. - PubMed
-
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. - PubMed
-
- Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

