Conditional inactivation of the CXCR4 receptor in osteoprecursors reduces postnatal bone formation due to impaired osteoblast development
- PMID: 21636574
- PMCID: PMC3143640
- DOI: 10.1074/jbc.M111.250985
Conditional inactivation of the CXCR4 receptor in osteoprecursors reduces postnatal bone formation due to impaired osteoblast development
Abstract
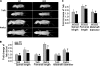
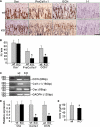
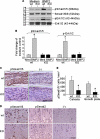
Cysteine (C)-X-C motif chemokine receptor 4 (CXCR4), the primary receptor for stromal cell-derived factor-1 (SDF-1), is involved in bone morphogenic protein 2 (BMP2)-induced osteogenic differentiation of mesenchymal progenitors. To target the in vivo function of CXCR4 in bone and explore the underlying mechanisms, we conditionally inactivated CXCR4 in osteoprecursors by crossing osterix (Osx)-Cre mice with floxed CXCR4 (CXCR4(fl/fl)) mice to generate knock-outs with CXCR4 deletion driven by the Osx promoter (Osx::CXCR4(fl/fl)). The Cre-mediated excision of CXCR4 occurred exclusively in bone of Osx::CXCR4(fl/fl) mice. When compared with littermate controls, Osx::CXCR4(fl/fl) mice developed smaller osteopenic skeletons as evidenced by reduced trabecular and cortical bone mass, lower bone mineral density, and a slower mineral apposition rate. In addition, Osx::CXCR4(fl/fl) mice displayed chondrocyte disorganization in the epiphyseal growth plate associated with decreased proliferation and collagen matrix syntheses. Moreover, mature osteoblast-related expression of type I collagen α1 and osteocalcin was reduced in bone of Osx::CXCR4(fl/fl) mice versus controls, suggesting that CXCR4 deficiency results in arrested osteoblast progression. Primary cultures for osteoblastic cells derived from Osx::CXCR4(fl/fl) mice also showed decreased proliferation and impaired osteoblast differentiation in response to BMP2 or BMP6 stimulation, and suppressed activation of intracellular BMP receptor-regulated Smads (R-Smads) and Erk1/2 was identified in CXCR4-deficient cells and bone tissues. These findings provide the first in vivo evidence that CXCR4 functions in postnatal bone development by regulating osteoblast development in cooperation with BMP signaling. Thus, CXCR4 acts as an endogenous signaling component necessary for bone formation.
Figures







References
-
- Shirozu M., Nakano T., Inazawa J., Tashiro K., Tada H., Shinohara T., Honjo T. (1995) Genomics 28, 495–500 - PubMed
-
- D'Apuzzo M., Rolink A., Loetscher M., Hoxie J. A., Clark-Lewis I., Melchers F., Baggiolini M., Moser B. (1997) Eur. J. Immunol. 27, 1788–1793 - PubMed
-
- Yu L., Cecil J., Peng S. B., Schrementi J., Kovacevic S., Paul D., Su E. W., Wang J. (2006) Gene 374, 174–179 - PubMed
-
- Zlotnik A., Yoshie O. (2000) Immunity 12, 121–127 - PubMed
-
- Nagasawa T., Hirota S., Tachibana K., Takakura N., Nishikawa S., Kitamura Y., Yoshida N., Kikutani H., Kishimoto T. (1996) Nature 382, 635–638 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

