Isotopomer profiling of Leishmania mexicana promastigotes reveals important roles for succinate fermentation and aspartate uptake in tricarboxylic acid cycle (TCA) anaplerosis, glutamate synthesis, and growth
- PMID: 21636575
- PMCID: PMC3149361
- DOI: 10.1074/jbc.M110.213553
Isotopomer profiling of Leishmania mexicana promastigotes reveals important roles for succinate fermentation and aspartate uptake in tricarboxylic acid cycle (TCA) anaplerosis, glutamate synthesis, and growth
Abstract
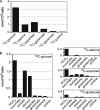
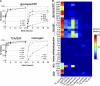
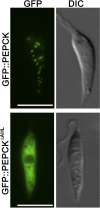
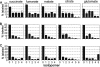
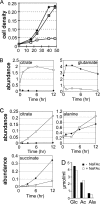
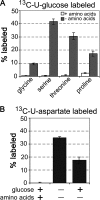
Leishmania parasites proliferate within nutritionally complex niches in their sandfly vector and mammalian hosts. However, the extent to which these parasites utilize different carbon sources remains poorly defined. In this study, we have followed the incorporation of various (13)C-labeled carbon sources into the intracellular and secreted metabolites of Leishmania mexicana promastigotes using gas chromatography-mass spectrometry and (13)C NMR. [U-(13)C]Glucose was rapidly incorporated into intermediates in glycolysis, the pentose phosphate pathway, and the cytoplasmic carbohydrate reserve material, mannogen. Enzymes involved in the upper glycolytic pathway are sequestered within glycosomes, and the ATP and NAD(+) consumed by these reactions were primarily regenerated by the fermentation of phosphoenolpyruvate to succinate (glycosomal succinate fermentation). The initiating enzyme in this pathway, phosphoenolpyruvate carboxykinase, was exclusively localized to the glycosome. Although some of the glycosomal succinate was secreted, most of the C4 dicarboxylic acids generated during succinate fermentation were further catabolized in the TCA cycle. A high rate of TCA cycle anaplerosis was further suggested by measurement of [U-(13)C]aspartate and [U-(13)C]alanine uptake and catabolism. TCA cycle anaplerosis is apparently needed to sustain glutamate production under standard culture conditions. Specifically, inhibition of mitochondrial aconitase with sodium fluoroacetate resulted in the rapid depletion of intracellular glutamate pools and growth arrest. Addition of high concentrations of exogenous glutamate alleviated this growth arrest. These findings suggest that glycosomal and mitochondrial metabolism in Leishmania promastigotes is tightly coupled and that, in contrast to the situation in some other trypanosomatid parasites, the TCA cycle has crucial anabolic functions.
Figures


References
-
- Murray H. W., Berman J. D., Davies C. R., Saravia N. G. (2005) Lancet 366, 1561–1577 - PubMed
-
- Opperdoes F. R., Coombs G. H. (2007) Trends Parasitol. 23, 149–158 - PubMed
-
- Saunders E. C., DE Souza D. P., Naderer T., Sernee M. F., Ralton J. E., Doyle M. A., Macrae J. I., Chambers J. L., Heng J., Nahid A., Likic V. A., McConville M. J. (2010) Parasitology 137, 1303–1313 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

