Accelerated maturation and abnormal morphology in the preterm neonatal kidney
- PMID: 21636639
- PMCID: PMC3137584
- DOI: 10.1681/ASN.2010121266
Accelerated maturation and abnormal morphology in the preterm neonatal kidney
Abstract
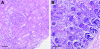
Nephrogenesis is ongoing at the time of birth for the majority of preterm infants, but whether postnatal renal development follows a similar trajectory to normal in utero growth is unknown. Here, we examined tissue collected at autopsy from 28 kidneys from preterm neonates, whose postnatal survival ranged from 2 to 68 days, including 6 that had restricted intrauterine growth. In addition, we examined kidneys from 32 still-born gestational controls. We assessed the width of the nephrogenic zone, number of glomerular generations, cross-sectional area of the renal corpuscle, and glomerular maturity and morphology. Renal maturation accelerated after preterm birth, with an increased number of glomerular generations and a decreased width of the nephrogenic zone in the kidneys of preterm neonates. Of particular concern, compared with gestational controls, preterm kidneys had a greater percentage of morphologically abnormal glomeruli and a significantly larger cross-sectional area of the renal corpuscle, suggestive of renal hyperfiltration. These observations suggest that the preterm kidney may have fewer functional nephrons, thereby increasing vulnerability to impaired renal function in both the early postnatal period and later in life.
Copyright © 2011 by the American Society of Nephrology
Figures

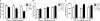
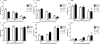



References
-
- Tracy S, Tracy M, Dean J, Laws P, Sullivan E: Spontaneous preterm birth of liveborn infants in women at low risk in Australia over 10 years: A population-based study. BJOG 114: 731–735, 2007 - PubMed
-
- Martin J, Hamilton B, Sutton P, Ventura S, Menacher F, Kirmeyer S: Births: Final data for 2004. Natl Vital Stat Rep 55: 1–101, 2006 - PubMed
-
- Fellman V, Hellstrom-Westas L, Norman M, Westgren M, Kallen K, Lagercrantz H, Marsal K, Serenius F, Wennergren M: One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 301: 2225–2233, 2009 - PubMed
-
- McCormick MC: The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med 312: 82–90, 1985 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

