Extracellular nucleotides and apyrases regulate stomatal aperture in Arabidopsis
- PMID: 21636723
- PMCID: PMC3149927
- DOI: 10.1104/pp.111.174466
Extracellular nucleotides and apyrases regulate stomatal aperture in Arabidopsis
Abstract
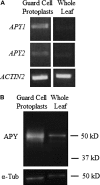
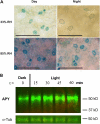
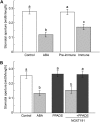
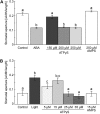
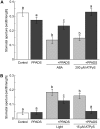
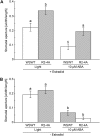
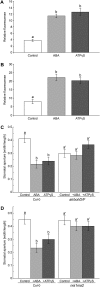
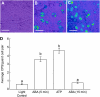
This study investigates the role of extracellular nucleotides and apyrase enzymes in regulating stomatal aperture. Prior data indicate that the expression of two apyrases in Arabidopsis (Arabidopsis thaliana), APY1 and APY2, is strongly correlated with cell growth and secretory activity. Both are expressed strongly in guard cell protoplasts, as determined by reverse transcription-polymerase chain reaction and immunoblot analyses. Promoter activity assays for APY1 and APY2 show that expression of both apyrases correlates with conditions that favor stomatal opening. Correspondingly, immunoblot data indicate that APY expression in guard cell protoplasts rises quickly when these cells are moved from darkness into light. Both short-term inhibition of ectoapyrase activity by polyclonal antibodies and long-term suppression of APY1 and APY2 transcript levels significantly disrupt normal stomatal behavior in light. Stomatal aperture shows a biphasic response to applied adenosine 5'-[γ-thio]triphosphate (ATPγS) or adenosine 5'-[β-thio] diphosphate, with lower concentrations inducing stomatal opening and higher concentrations inducing closure. Equivalent concentrations of adenosine 5'-O-thiomonophosphate have no effect on aperture. Two mammalian purinoceptor inhibitors block ATPγS- and adenosine 5'-[β-thio] diphosphate-induced opening and closing and also partially block the ability of abscisic acid to induce stomatal closure and of light to induce stomatal opening. Treatment of epidermal peels with ATPγS induces increased levels of nitric oxide and reactive oxygen species, and genetically suppressing the synthesis of these agents blocks the effects of nucleotides on stomatal aperture. A luciferase assay indicates that treatments that induce either the closing or opening of stomates also induce the release of ATP from guard cells. These data favor the novel conclusion that ectoapyrases and extracellular nucleotides play key roles in regulating stomatal functions.
Figures

References
-
- Barbour MM, Buckley TN. (2007) The stomatal response to evaporative demand persists at night in Ricinus communis plants with high nocturnal conductance. Plant Cell Environ 30: 711–721 - PubMed
-
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 - PubMed
-
- Burnstock G. (2008) Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7: 575–590 - PubMed
-
- Clark G, Roux SJ. (2009) Extracellular nucleotides: ancient signaling molecules. Plant Sci 177: 239–244
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

