Intrinsic functional architecture predicts electrically evoked responses in the human brain
- PMID: 21636787
- PMCID: PMC3121855
- DOI: 10.1073/pnas.1019750108
Intrinsic functional architecture predicts electrically evoked responses in the human brain
Erratum in
- Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):17234
Abstract
Adaptive brain function is characterized by dynamic interactions within and between neuronal circuits, often occurring at the time scale of milliseconds. These complex interactions between adjacent and noncontiguous brain areas depend on a functional architecture that is maintained even in the absence of input. Functional MRI studies carried out during rest (R-fMRI) suggest that this architecture is represented in low-frequency (<0.1 Hz) spontaneous fluctuations in the blood oxygen level-dependent signal that are correlated within spatially distributed networks of brain areas. These networks, collectively referred to as the brain's intrinsic functional architecture, exhibit a remarkable correspondence with patterns of task-evoked coactivation as well as maps of anatomical connectivity. Despite this striking correspondence, there is no direct evidence that this intrinsic architecture forms the scaffold that gives rise to faster processes relevant to information processing and seizure spread. Here, we demonstrate that the spatial distribution and magnitude of temporally correlated low-frequency fluctuations observed with R-fMRI during rest predict the pattern and magnitude of corticocortical evoked potentials elicited within 500 ms after single-pulse electrical stimulation of the cerebral cortex with intracranial electrodes. Across individuals, this relationship was found to be independent of the specific regions and functional systems probed. Our findings bridge the immense divide between the temporal resolutions of these distinct measures of brain function and provide strong support for the idea that the low-frequency signal fluctuations observed with R-fMRI maintain and update the intrinsic architecture underlying the brain's repertoire of functional responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

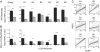

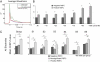
References
-
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. - PubMed
-
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. - PubMed
-
- He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Curr Opin Neurol. 2007;20:655–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

