Placental growth factor regulates cardiac adaptation and hypertrophy through a paracrine mechanism
- PMID: 21636802
- PMCID: PMC3146170
- DOI: 10.1161/CIRCRESAHA.111.240820
Placental growth factor regulates cardiac adaptation and hypertrophy through a paracrine mechanism
Abstract
Rationale: Paracrine growth factor-mediated crosstalk between cardiac myocytes and nonmyocytes in the heart is critical for programming adaptive cardiac hypertrophy in which myocyte size, capillary density, and the extracellular matrix function coordinately.
Objective: To examine the role that placental growth factor (PGF) plays in the heart as a paracrine regulator of cardiac adaptation to stress stimulation.
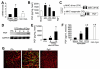
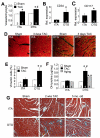
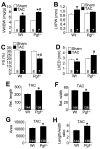
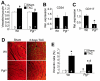
Methods and results: PGF is induced in the heart after pressure-overload stimulation, where it is expressed in both myocytes and nonmyocytes. We generated cardiac-specific and adult inducible PGF-overexpressing transgenic mice and analyzed Pgf(-/-) mice to examine the role that this factor plays in cardiac disease and paracrine signaling. Although PGF transgenic mice did not have a baseline phenotype or a change in capillary density, they did exhibit a greater cardiac hypertrophic response, a greater increase in capillary density, and increased fibroblast content in the heart in response to pressure-overload stimulation. PGF transgenic mice showed a more adaptive type of cardiac growth that was protective against signs of failure with pressure overload and neuroendocrine stimulation. Antithetically, Pgf(-/-) mice rapidly died of heart failure within 1 week of pressure overload, they showed an inability to upregulate angiogenesis, and they showed significantly less fibroblast activity in the heart. Mechanistically, we show that PGF does not have a direct effect on cardiomyocytes but works through endothelial cells and fibroblasts by inducing capillary growth and fibroblast proliferation, which secondarily support greater cardiac hypertrophy through intermediate paracrine growth factors such as interleukin-6.
Conclusions: PGF is a secreted factor that supports hypertrophy and cardiac function during pressure overload by affecting endothelial cells and fibroblasts that in turn stimulate and support the myocytes through additional paracrine factors.
Figures


References
-
- Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. - PubMed
-
- Lorell BH, Carabello BA. Left ventricular hypertrophy: Pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. - PubMed
-
- Frey N, Olson EN. Cardiac hypertrophy: The good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. - PubMed
-
- Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

