Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation
- PMID: 21637782
- PMCID: PMC3102739
- DOI: 10.1371/journal.pone.0020540
Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation
Abstract
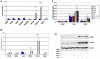
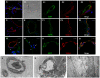
Here, we identify CD44(+)CD90(+)CD73(+)CD34(-)CD45(-) cells within the adult human arterial adventitia with properties of multipotency which were named vascular wall-resident multipotent stem cells (VW-MPSCs). VW-MPSCs exhibit typical mesenchymal stem cell characteristics including cell surface markers in immunostaining and flow cytometric analyses, and differentiation into adipocytes, chondrocytes and osteocytes under culture conditions. Particularly, TGFß1 stimulation up-regulates smooth muscle cell markers in VW-MPSCs. Using fluorescent cell labelling and co-localisation studies we show that VW-MPSCs differentiate to pericytes/smooth muscle cells which cover the wall of newly formed endothelial capillary-like structures in vitro. Co-implantation of EGFP-labelled VW-MPSCs and human umbilical vein endothelial cells into SCID mice subcutaneously via Matrigel results in new vessels formation which were covered by pericyte- or smooth muscle-like cells generated from implanted VW-MPSCs. Our results suggest that VW-MPSCs are of relevance for vascular morphogenesis, repair and self-renewal of vascular wall cells and for local capacity of neovascularization in disease processes.
Conflict of interest statement
Figures





References
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
-
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–32. - PubMed
-
- Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

