EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate
- PMID: 21638067
- PMCID: PMC3155049
- DOI: 10.1007/s10456-011-9217-1
EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate
Abstract
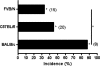
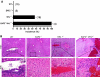
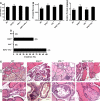
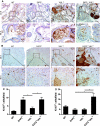
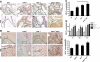
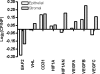
Von Hippel-Lindau (VHL) disease results from the inactivation of the VHL gene and is characterized by highly vascular tumors. A consequence of VHL loss is the stabilization of hypoxia-inducible factor (HIF) alpha subunits and increased expression of HIF target genes, which include pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF). In mice, homozygous deletion of VHL is embryonic lethal due to vascular abnormalities in the placenta; and, VHL(+/-) mice develop proliferative vascular lesions in several major organs, most prominently the liver. Loss of ELL-associated factor (EAF2) in murine models has also been shown to induce increased vascular density in the liver as well as the prostate. Previously, EAF2 was determined to be a binding partner of VHL and loss of EAF2 induced a reduction in pVHL levels and an increase in hypoxia induced factor 1α (HIF1α) levels in vitro. Here we characterized the cooperative effects of VHL- and EAF2-deficiency on angiogenesis in the liver and prostate of male mice. VHL deficiency consistently increased the incidence of hepatic vascular lesions across three mouse strains. These vascular lesions where characterized by an increase in microvessel density, and staining intensity of VHL target proteins HIF1α and VEGF. EAF2(-/-)VHL(+/-) mice had increased incidence of proliferative hepatic vascular lesions (4/4) compared to VHL(+/-) (10/18) and EAF2(-/-) (0/5) mice. Prostates of EAF2(-/-)VHL(+/-) mice also displayed an increase in microvessel density, as well as stromal inflammation and prostatic intraepithelial neoplasia. These results suggest that cooperation of VHL and EAF2 may be critical for angiogenic regulation of the liver and prostate, and concurrent loss of these two tumor suppressors may result in a pro-angiogenic phenotype.
Figures



Similar articles
-
U19/Eaf2 binds to and stabilizes von hippel-lindau protein.Cancer Res. 2009 Mar 15;69(6):2599-606. doi: 10.1158/0008-5472.CAN-08-2595. Epub 2009 Mar 3. Cancer Res. 2009. PMID: 19258512 Free PMC article.
-
Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice.Mol Cell Biol. 2005 Apr;25(8):3163-72. doi: 10.1128/MCB.25.8.3163-3172.2005. Mol Cell Biol. 2005. PMID: 15798202 Free PMC article.
-
Action of hypoxia-inducible factor in liver and kidney from mice with Pax8-rtTA-based deletion of von Hippel-Lindau protein.Acta Physiol (Oxf). 2013 Mar;207(3):565-76. doi: 10.1111/apha.12058. Acta Physiol (Oxf). 2013. PMID: 23384425
-
Von hippel-lindau disease: a genetic and clinical review.Semin Ophthalmol. 2013 Sep-Nov;28(5-6):377-86. doi: 10.3109/08820538.2013.825281. Semin Ophthalmol. 2013. PMID: 24138046 Review.
-
The von Hippel-Lindau tumor suppressor gene.Exp Cell Res. 2001 Mar 10;264(1):117-25. doi: 10.1006/excr.2000.5139. Exp Cell Res. 2001. PMID: 11237528 Review.
Cited by
-
E-cadherin deficiency promotes prostate macrophage inflammation and bladder overactivity in aged male mice.Aging (Albany NY). 2022 Mar 31;14(7):2945-2965. doi: 10.18632/aging.203994. Epub 2022 Mar 31. Aging (Albany NY). 2022. PMID: 35361739 Free PMC article.
-
Gene Expression Profiling Reveals Regulation of ERK Phosphorylation by Androgen-Induced Tumor Suppressor U19/EAF2 in the Mouse Prostate.Cancer Microenviron. 2013 Dec;6(3):247-61. doi: 10.1007/s12307-013-0132-4. Epub 2013 Feb 26. Cancer Microenviron. 2013. PMID: 23440596 Free PMC article.
-
EAF2 and p53 Co-Regulate STAT3 Activation in Prostate Cancer.Neoplasia. 2018 Apr;20(4):351-363. doi: 10.1016/j.neo.2018.01.011. Epub 2018 Mar 6. Neoplasia. 2018. PMID: 29518696 Free PMC article.
-
Characterization of prostate macrophage heterogeneity, foam cell markers, and CXCL17 upregulation in a mouse model of steroid hormone imbalance.Sci Rep. 2024 Sep 9;14(1):21029. doi: 10.1038/s41598-024-71137-4. Sci Rep. 2024. PMID: 39251671 Free PMC article.
-
EZH2 promotes metabolic reprogramming in glioblastomas through epigenetic repression of EAF2-HIF1α signaling.Oncotarget. 2016 Jul 19;7(29):45134-45143. doi: 10.18632/oncotarget.9761. Oncotarget. 2016. PMID: 27259264 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

