Molecular mechanisms of Staphylococcus aureus iron acquisition
- PMID: 21639791
- PMCID: PMC3807827
- DOI: 10.1146/annurev-micro-090110-102851
Molecular mechanisms of Staphylococcus aureus iron acquisition
Abstract
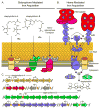
The unique redox potential of iron makes it an ideal cofactor in diverse biochemical reactions. Iron is therefore vital for the growth and proliferation of nearly all organisms, including pathogenic bacteria. Vertebrates sequester excess iron within proteins in order to alleviate toxicity and restrict the amount of free iron available for invading pathogens. Restricting the growth of infectious microorganisms by sequestering essential nutrients is referred to as nutritional immunity. In order to circumvent nutritional immunity, bacterial pathogens have evolved elegant systems that allow for the acquisition of iron during infection. The gram-positive extracellular pathogen Staphylococcus aureus is a commensal organism that can cause severe disease when it gains access to underlying tissues. Iron acquisition is required for S. aureus colonization and subsequent pathogenesis. Herein we review the strategies S. aureus employs to obtain iron through the production of siderophores and the consumption of host heme.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

