The serotonergic anatomy of the developing human medulla oblongata: implications for pediatric disorders of homeostasis
- PMID: 21640183
- PMCID: PMC3134154
- DOI: 10.1016/j.jchemneu.2011.05.004
The serotonergic anatomy of the developing human medulla oblongata: implications for pediatric disorders of homeostasis
Abstract
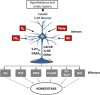
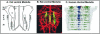
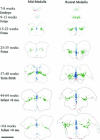
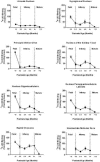
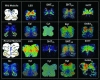
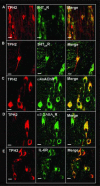
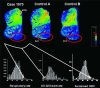
The caudal serotonergic (5-HT) system is a critical component of a medullary "homeostatic network" that regulates protective responses to metabolic stressors such as hypoxia, hypercapnia, and hyperthermia. We define anatomically the caudal 5-HT system in the human medulla as 5-HT neuronal cell bodies located in the raphé (raphé obscurus, raphé magnus, and raphé pallidus), extra-raphé (gigantocellularis, paragigantocellularis lateralis, intermediate reticular zone, lateral reticular nucleus, and nucleus subtrigeminalis), and ventral surface (arcuate nucleus). These 5-HT neurons are adjacent to all of the respiratory- and autonomic-related nuclei in the medulla where they are positioned to modulate directly the responses of these effector nuclei. In the following review, we highlight the topography and development of the caudal 5-HT system in the human fetus and infant, and its inter-relationships with nicotinic, GABAergic, and cytokine receptors. We also summarize pediatric disorders in early life which we term "developmental serotonopathies" of the caudal (as well as rostral) 5-HT domain and which are associated with homeostatic imbalances. The delineation of the development and organization of the human caudal 5-HT system provides the critical foundation for the neuropathologic elucidation of its disorders directly in the human brain.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
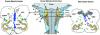



References
-
- Adamsen D, Meili D, Blau N, Thony B, Ramaekers V. Autism associated with low 5-hydroxyindolacetic acid in CSF and the heterozygous SLC6A4 gene Gly56Ala plus 5-HTTLPR L/L promoter variants. Mol Genet Metab. 2011;102:368–373. - PubMed
-
- Akefeldt A, Ekman R, Gillberg C, Mansson JE. Cerebrospinal fluid monoamines in Prader-Willi syndrome. Biol Psychiatry. 1998;44:1321–1328. - PubMed
-
- Akefeldt A, Mansson JE. Is monoamine oxidase activity elevated in Prader-Willi syndrome? Eur Child Adolesc Psychiatry. 1998;7:163–165. - PubMed
-
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

