MR perfusion imaging in acute ischemic stroke
- PMID: 21640299
- PMCID: PMC3135980
- DOI: 10.1016/j.nic.2011.02.007
MR perfusion imaging in acute ischemic stroke
Abstract
Magnetic resonance (MR) perfusion imaging offers the potential for measuring brain perfusion in acute stroke patients, at a time when treatment decisions based on these measurements may affect outcomes dramatically. Rapid advancements in both acute stroke therapy and perfusion imaging techniques have resulted in continuing redefinition of the role that perfusion imaging should play in patient management. This review discusses the basic pathophysiology of acute stroke, the utility of different kinds of perfusion images, and research on the continually evolving role of MR perfusion imaging in acute stroke care.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
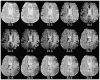
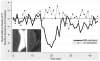

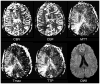

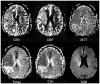
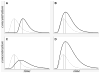
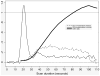
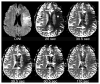
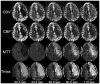

References
-
- Rosen BR, Belliveau JW, Chien D. Perfusion imaging by nuclear magnetic resonance. Magn Reson Q. 1989;5:263–281. - PubMed
-
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. - PubMed
-
- Grubb RL, Jr, Phelps ME, Ter-Pogossian MM. Regional cerebral blood volume in humans. X-ray fluorescence studies. Arch Neurol. 1973;28:38–44. - PubMed
-
- Grubb RL, Jr, Phelps ME, Raichle ME, Ter-Pogossian MM. The effects of arterial blood pressure on the regional cerebral blood volume by x-ray fluorescence. Stroke. 1973;4:390–399. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

