Spontaneous lung dysfunction and fibrosis in mice lacking connexin 40 and endothelial cell connexin 43
- PMID: 21641379
- PMCID: PMC3124229
- DOI: 10.1016/j.ajpath.2011.02.045
Spontaneous lung dysfunction and fibrosis in mice lacking connexin 40 and endothelial cell connexin 43
Abstract
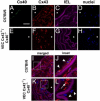
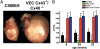
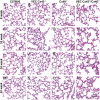
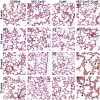
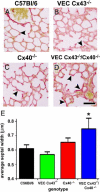
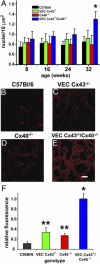
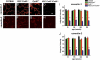
Gap junction proteins (connexins) facilitate intercellular communication and serve several roles in regulation of tissue function and remodeling. To examine the physiologic effects of depleting two prominent endothelial connexins, Cx40 and Cx43, transgenic mice were generated by breeding Cx40-deficient mice (Cx40(-/-)) with a vascular endothelial cell (VEC)-specific Cx43-deficient mouse strain (VEC Cx43(-/-)) to produce double-connexin knockout mice (VEC Cx43(-/-)/Cx40(-/-)). The life span in VEC Cx43(-/-)/Cx40(-/-) mice was dramatically shortened, which correlated with severe spontaneous lung abnormalities as the mice aged including increased fibrosis, aberrant alveolar remodeling, and increased lung fibroblast content. Moreover, VEC Cx43(-/-)/Cx40(-/-) mice exhibited cardiac hypertrophy and hypertension. Because VEC Cx43(-/-)/Cx40(-/-) mice demonstrated phenotypic hallmarks that were remarkably similar to those in mice deficient in caveolin-1, pulmonary caveolin expression was examined. Lungs from VEC Cx43(-/-)/Cx40(-/-) mice demonstrated significantly decreased expression of caveolin-1 and caveolin-2. This suggests that expression of caveolin-1 may be linked to expression of Cx40 and endothelial Cx43. Moreover, the phenotype of caveolin-1(-/-) mice and VEC Cx43(-/-)/Cx40(-/-) mice may arise via a common mechanism.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Sohl G., Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. - PubMed
-
- Koval M. Sharing signals: connecting lung epithelial cells with gap junction channels. Am J Physiol Lung Cell Mol Physiol. 2002;283:L875–L893. - PubMed
-
- Abraham V., Chou M.L., George P., Pooler P., Zaman A., Savani R.C., Koval M. Heterocellular gap junctional communication between alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1085–L1093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

