Apoptosis-like cell death induction and aberrant fibroblast properties in human incisional hernia fascia
- PMID: 21641387
- PMCID: PMC3124017
- DOI: 10.1016/j.ajpath.2011.02.044
Apoptosis-like cell death induction and aberrant fibroblast properties in human incisional hernia fascia
Abstract
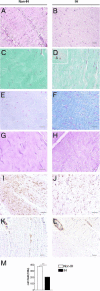
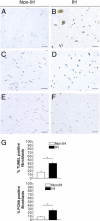
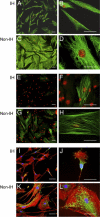
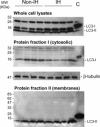
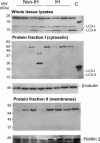
Incisional hernia often occurs following laparotomy and can be a source of serious problems. Although there is evidence that a biological cause may underlie its development, the mechanistic link between the local tissue microenvironment and tissue rupture is lacking. In this study, we used matched tissue-based and in vitro primary cell culture systems to examine the possible involvement of fascia fibroblasts in incisional hernia pathogenesis. Fascia biopsies were collected at surgery from incisional hernia patients and non-incisional hernia controls. Tissue samples were analyzed by histology and immunoblotting methods. Fascia primary fibroblast cultures were assessed at morphological, ultrastructural, and functional levels. We document tissue and fibroblast loss coupled to caspase-3 activation and induction of apoptosis-like cell-death mechanisms in incisional hernia fascia. Alterations in cytoskeleton organization and solubility were also observed. Incisional hernia fibroblasts showed a consistent phenotype throughout early passages in vitro, which was characterized by significantly enhanced cell proliferation and migration, reduced adhesion, and altered cytoskeleton properties, as compared to non-incisional hernia fibroblasts. Moreover, incisional hernia fibroblasts displayed morphological and ultrastructural alterations compatible with autophagic processes or lysosomal dysfunction, together with enhanced sensitivity to proapoptotic challenges. Overall, these data suggest an ongoing complex interplay of cell death induction, aberrant fibroblast function, and tissue loss in incisional hernia fascia, which may significantly contribute to altered matrix maintenance and tissue rupture in vivo.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Muysoms F.E., Miserez M., Berrevoet F., Campanelli G., Champault G.G., Chelala E., Dietz U.A., Eker H.H., El Nakadi I., Hauters P., Hidalgo Pascual M., Hoeferlin A., Klinge U., Montgomery A., Simmermacher R.K., Simons M.P., Smietański M., Sommeling C., Tollens T., Vierendeels T., Kingsnorth A., Muysoms F.E., Miserez M., Berrevoet F. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13:407–414. - PMC - PubMed
-
- Arbos M.A., Ferrando J.M., Quiles M.T., Vidal J., López-Cano M., Gil J., Manero J.M., Peña J., Huguet P., Schwartz-Riera S., Reventós J., Armengol M. Improved surgical mesh integration into the rat abdominal wall with arginine administration. Biomaterials. 2006;27:758–768. - PubMed
-
- Yahchouchy-Chouillard E., Aura T., Picone O., Etienne J.C., Fingerhut A. Incisional hernias: I. Related risk factors. Dig Surg. 2003;20:3–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

