Human leukocyte antigen class II transgenic mouse model unmasks the significant extrahepatic pathology in toxic shock syndrome
- PMID: 21641398
- PMCID: PMC3124354
- DOI: 10.1016/j.ajpath.2011.02.033
Human leukocyte antigen class II transgenic mouse model unmasks the significant extrahepatic pathology in toxic shock syndrome
Abstract
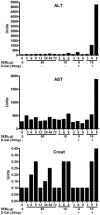
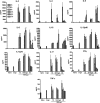
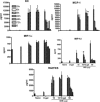
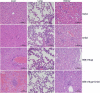
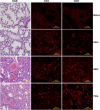
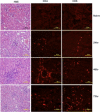
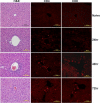
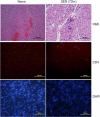
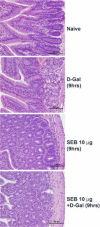
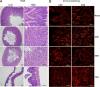
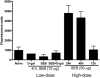
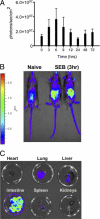
Among the exotoxins produced by Staphylococcus aureus and Streptococcus pyogenes, the superantigens (SAgs) are the most potent T-cell activators known to date. SAgs are implicated in several serious diseases including toxic shock syndrome (TSS), Kawasaki disease, and sepsis. However, the immunopathogenesis of TSS and other diseases involving SAgs are still not completely understood. The commonly used conventional laboratory mouse strains do not respond robustly to SAgs in vivo. Therefore, they must be artificially rendered susceptible to TSS by using sensitizing agents such as d-galactosamine (d-galN), which skews the disease exclusively to the liver and, hence, is not representative of the disease in humans. SAg-induced TSS was characterized using transgenic mice expressing HLA class II molecules that are extremely susceptible to TSS without d-galN. HLA-DR3 transgenic mice recapitulated TSS in humans with extensive multiple-organ inflammation affecting the lung, liver, kidneys, heart, and small intestines. Heavy infiltration with T lymphocytes (both CD4(+) and CD8+), neutrophils, and macrophages was noted. In particular, the pathologic changes in the small intestines were extensive and accompanied by significantly altered absorptive functions of the enterocytes. In contrast to massive liver failure alone in the d-galN sensitization model of TSS, findings of the present study suggest that gut dysfunction might be a key pathogenic event that leads to high morbidity and mortality in humans with TSS.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Brooks G.F., Carroll K.C., Butel J.S., Morse S.A., editors. The staphylococci. Jawetz, Melnick, & Adelberg’s Medical Microbiology. ed 25. The McGraw-Hill Companies, Inc; New York: 2010. ch 13.
-
- Manders S.M. Toxin-mediated streptococcal and staphylococcal disease. J Am Acad Dermatol. 1998;39:383–398. - PubMed
-
- Fraser D.J., Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–243. - PubMed
-
- Li H., Llera A., Malchiodi E.L., Mariuzza R.A. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

