The circadian clock interacts with metabolic physiology to influence reproductive fitness
- PMID: 21641546
- PMCID: PMC3152999
- DOI: 10.1016/j.cmet.2011.05.001
The circadian clock interacts with metabolic physiology to influence reproductive fitness
Abstract
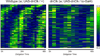
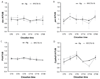
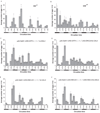
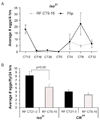
Circadian rhythms are regulated by a synchronized system of central and peripheral clocks. Here, we show that a clock in the Drosophila fat body drives rhythmic expression of genes involved in metabolism, detoxification, the immune response, and steroid hormone regulation. Some of these genes cycle even when the fat body clock is disrupted, indicating that they are regulated by exogenous factors. Food is an important stimulus, as limiting food availability to a 6 hr interval each day drives rhythmic expression of genes in the fat body. Restricting food to a time of day when consumption is typically low desynchronizes internal rhythms because it alters the phase of rhythmic gene expression in the fat body without affecting the brain clock. Flies maintained on this paradigm produce fewer eggs than those restricted to food at the normal time. These data suggest that desynchrony of endogenous rhythms, caused by aberrant feeding patterns, affects reproductive fitness.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Feeding mistiming decreases reproductive fitness in flies.Cell Metab. 2011 Jun 8;13(6):613-4. doi: 10.1016/j.cmet.2011.05.003. Cell Metab. 2011. PMID: 21641540 Free PMC article.
References
-
- Allemand R. Influence of light condition modification on the circadian rhythm of vitellogenesis and ovulation in Drosophila melanogaster. J Insect Physiol. 1976;22:1075–1080. - PubMed
-
- Bownes M. The regulation of the yolk protein genes, a family of sex differentiation genes in Drosophila melanogaster. Bioessays. 1994;16:745–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 NS048471/NS/NINDS NIH HHS/United States
- 1R01HL097800/HL/NHLBI NIH HHS/United States
- R01 NS048471/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- 1R56NS048471/NS/NINDS NIH HHS/United States
- R01 NS054794/NS/NINDS NIH HHS/United States
- R01 HL097800/HL/NHLBI NIH HHS/United States
- 1R01NS048471/NS/NINDS NIH HHS/United States
- P50 MH074924/MH/NIMH NIH HHS/United States
- P01 AG017628/AG/NIA NIH HHS/United States
- T32 AG000255/AG/NIA NIH HHS/United States
- T32-AG000255/AG/NIA NIH HHS/United States
- 1R01NS054794/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

