Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype
- PMID: 21641555
- PMCID: PMC3148136
- DOI: 10.1016/j.cmet.2011.05.002
Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype
Abstract
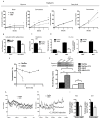
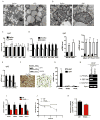
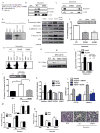
While fatty acids (FAs) released by white adipose tissue (WAT) provide energy for other organs, lipolysis is also critical in brown adipose tissue (BAT), generating FAs for oxidation and UCP-1 activation for thermogenesis. Here we show that adipose-specific ablation of desnutrin/ATGL in mice converts BAT to a WAT-like tissue. These mice exhibit severely impaired thermogenesis with increased expression of WAT-enriched genes but decreased BAT genes, including UCP-1 with lower PPARα binding to its promoter, revealing the requirement of desnutrin-catalyzed lipolysis for maintaining a BAT phenotype. We also show that desnutrin is phosphorylated by AMPK at S406, increasing TAG hydrolase activity, and provide evidence for increased lipolysis by AMPK phosphorylation of desnutrin in adipocytes and in vivo. Despite adiposity and impaired BAT function, desnutrin-ASKO mice have improved hepatic insulin sensitivity with lower DAG levels. Overall, desnutrin is phosphorylated/activated by AMPK to increase lipolysis and brings FA oxidation and UCP-1 induction for thermogenesis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. - PubMed
-
- Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276:1486–1493. - PubMed
-
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

