Caveolin-1 modulates TGF-β1 signaling in cardiac remodeling
- PMID: 21641995
- PMCID: PMC4489541
- DOI: 10.1016/j.matbio.2011.05.003
Caveolin-1 modulates TGF-β1 signaling in cardiac remodeling
Abstract
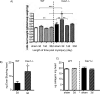
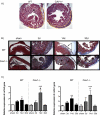
The cardiac response to myocardial injury includes fibrotic and hypertrophic processes and a key mediator in this response is transforming growth factor-β1 (TGF-β1). Caveolin-1 (cav1), the main structural protein of caveolae, is an inhibitor of the TGF-β1 signaling pathway. To examine the role of cav1 in cardiac repair, cav1 deficient (Cav1(-/-)) and wild type (WT) mice were subjected to cryoinjury of the left ventricle (LV). At baseline the two groups exhibited no inflammation, similar collagen content, and similar cardiac function. After injury, Cav1(-/-) animals displayed enhanced TGF-β1 signaling, as reflected by a 3-fold increase in the activation of the Smad2-dependent pathway and more widespread collagen deposition in the heart. Qualitative and quantitative analyses indicated that collagen deposition peaked in the WT LV 14days after injury, accompanied by increased mRNA abundance for procol1a2 (2-fold) and procol3a1 (3-fold). Collagen deposition was further enhanced in Cav1(-/-) mice, which was accompanied by reduced expression of matrix metalloproteinases MMP-8 (3-fold) and -13 mRNA (2-fold). The levels of expression of inflammatory markers of acute phase were similar between the strains However, macrophage clearance in the damaged region was delayed in Cav1(-/-) mice. We observed a 4-fold decrease in collagen deposition in Cav1(-/-) mice injected with a cav1 scaffolding domain peptide (CSD) and a 2-fold decrease in WT mice treated with the CSD. We conclude that cav1 has a direct role in reducing TGF-β1 signaling and as such might be an appropriate target for therapies to influence cardiac remodeling.
Copyright © 2011 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Figures







References
-
- Augustus AS, Buchanan J, Gutman E, Rengo G, Pestell RG, et al. Hearts lacking caveolin-1 develop hypertrophy with normal cardiac substrate metabolism. Cell Cycle. 2008;7:2509–2518. - PubMed
-
- Barth K, Gentsch M, Blasche R, Pfuller A, Parshyna I, et al. Distribution of caveolin-1 and connexin43 in normal and injured alveolar epithelial R3/1 cells. Histochem Cell Biol. 2005;123:239–247. - PubMed
-
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

