Nitric oxide regulates pulmonary vascular smooth muscle cell expression of the inducible cAMP early repressor gene
- PMID: 21642009
- PMCID: PMC3466086
- DOI: 10.1016/j.niox.2011.05.006
Nitric oxide regulates pulmonary vascular smooth muscle cell expression of the inducible cAMP early repressor gene
Abstract
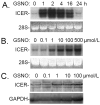
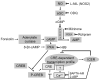
Nitric oxide (NO) regulates vascular smooth muscle cell (VSMC) structure and function, in part by activating soluble guanylate cyclase (sGC) to synthesize cGMP. The objective of this study was to further characterize the signaling mechanisms by which NO regulates VSMC gene expression using transcription profiling. DNA microarrays were hybridized with RNA extracted from rat pulmonary artery smooth muscle cells (RPaSMC) exposed to the NO donor compound, S-nitroso-glutathione (GSNO). Many of the genes, whose expression was induced by GSNO, contain a cAMP-response element (CRE), of which one encoded the inducible cAMP early repressor (ICER). sGC and cAMP-dependent protein kinase, but not cGMP-dependent protein kinase, were required for NO-mediated phosphorylation of CRE-binding protein (CREB) and induction of ICER gene expression. Expression of a dominant-negative CREB in RPaSMC prevented the NO-mediated induction of CRE-dependent gene transcription and ICER gene expression. Pre-treatment of RPaSMC with the intracellular calcium (Ca(2+)) chelator, BAPTA-AM, blocked the induction of ICER gene expression by GSNO. The store-operated Ca(2+) channel inhibitors, 2-ABP, and SKF-96365, reduced the GSNO-mediated increase in ICER mRNA levels, while 2-ABP did not inhibit GSNO-induced CREB phosphorylation. Our results suggest that induction of ICER gene expression by NO requires both CREB phosphorylation and Ca(2+) signaling. Transcription profiling of RPaSMC exposed to GSNO revealed important roles for sGC, PKA, CREB, and Ca(2+) in the regulation of gene expression by NO. The induction of ICER in GSNO-treated RPaSMC highlights a novel cross-talk mechanism between cGMP and cAMP signaling pathways.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

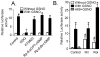



References
-
- Eigenthaler M, Lohmann SM, Walter U, Pilz RB. Signal transduction by cGMP-dependent protein kinases and their emerging roles in the regulation of cell adhesion and gene expression. Rev Physiol Biochem Pharmacol. 1999;135:173–209. - PubMed
-
- Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–91. - PubMed
-
- Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993;1178:153–75. - PubMed
-
- Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, Kleppisch T, Ruth P, Hofmann F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000;87:825–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

