Paracrine and endocrine effects of adipose tissue on cancer development and progression
- PMID: 21642230
- PMCID: PMC3369575
- DOI: 10.1210/er.2010-0030
Paracrine and endocrine effects of adipose tissue on cancer development and progression
Abstract
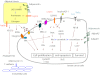
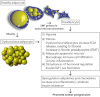
The past few years have provided substantial evidence for the vital role of the local tumor microenvironment for various aspects of tumor progression. With obesity and its pathophysiological sequelae still on the rise, the adipocyte is increasingly moving center stage in the context of tumor stroma-related studies. To date, we have limited insight into how the systemic metabolic changes associated with obesity and the concomitant modification of the paracrine and endocrine panel of stromal adipocyte-derived secretory products ("adipokines") influence the incidence and progression of obesity-related cancers. Here, we discuss the role of adipocyte dysfunction associated with obesity and its potential impact on cancer biology.
Figures


References
-
- Rajala MW, Scherer PE. 2003. Minireview: the adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 144:3765–3773 - PubMed
-
- Lionetti L, Mollica MP, Lombardi A, Cavaliere G, Gifuni G, Barletta A. 2009. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr Metab Cardiovasc Dis 19:146–152 - PubMed
-
- Blüher M. 2009. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes 117:241–250 - PubMed
-
- Calle EE, Kaaks R. 2004. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579–591 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

