eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity
- PMID: 21642378
- PMCID: PMC3326601
- DOI: 10.1530/JOE-11-0083
eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity
Abstract
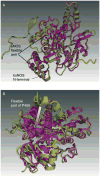
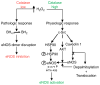
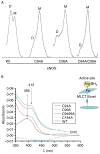
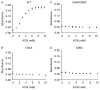
Rather than being a constitutive enzyme as was first suggested, endothelial nitric oxide synthase (eNOS) is dynamically regulated at the transcriptional, posttranscriptional, and posttranslational levels. This review will focus on how changes in eNOS function are conferred by various posttranslational modifications. The latest knowledge regarding eNOS targeting to the plasma membrane will be discussed as the role of protein phosphorylation as a modulator of catalytic activity. Furthermore, new data are presented that provide novel insights into how disruption of the eNOS dimer prevents eNOS uncoupling and the production of superoxide under conditions of elevated oxidative stress and identifies a novel regulatory region we have termed the 'flexible arm'.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures



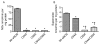

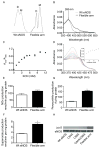
Comment in
-
Endothelial nitric oxide synthase activation and nitric oxide function: new light through old windows.J Endocrinol. 2011 Sep;210(3):239-41. doi: 10.1530/JOE-11-0216. J Endocrinol. 2011. PMID: 21824899
References
-
- Aschner JL, Foster SL, Kaplowitz M, Zhang Y, Zeng H, Fike CD. Heat shock protein 90 modulates endothelial nitric oxide synthase activity and vascular reactivity in the newborn piglet pulmonary circulation. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2007;292:L1515–L1525. doi: 10.1152/ajplung.00252.2006. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL084739/HL/NHLBI NIH HHS/United States
- R01 HL070061/HL/NHLBI NIH HHS/United States
- R01 HL092446/HL/NHLBI NIH HHS/United States
- R01 HL084739/HL/NHLBI NIH HHS/United States
- R21HD057406/HD/NICHD NIH HHS/United States
- HL67841/HL/NHLBI NIH HHS/United States
- R01 HL072123/HL/NHLBI NIH HHS/United States
- R01 HL067841/HL/NHLBI NIH HHS/United States
- HL60190/HL/NHLBI NIH HHS/United States
- R01 HL085827/HL/NHLBI NIH HHS/United States
- R01 HL060190/HL/NHLBI NIH HHS/United States
- HL085827/HL/NHLBI NIH HHS/United States
- R21 HD057406/HD/NICHD NIH HHS/United States
- HL72123/HL/NHLBI NIH HHS/United States
- 5T32HL06699/HL/NHLBI NIH HHS/United States
- HL70061/HL/NHLBI NIH HHS/United States
- HL092446/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

