Permeabilization of the mitochondrial outer membrane by Bax/truncated Bid (tBid) proteins as sensitized by cardiolipin hydroperoxide translocation: mechanistic implications for the intrinsic pathway of oxidative apoptosis
- PMID: 21642428
- PMCID: PMC3143596
- DOI: 10.1074/jbc.M110.188516
Permeabilization of the mitochondrial outer membrane by Bax/truncated Bid (tBid) proteins as sensitized by cardiolipin hydroperoxide translocation: mechanistic implications for the intrinsic pathway of oxidative apoptosis
Abstract
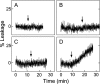
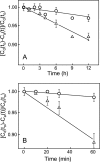
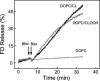
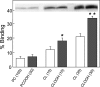
Cytochrome c (cyt c) release upon oxidation of cardiolipin (CL) in the mitochondrial inner membrane (IM) under oxidative stress occurs early in the intrinsic apoptotic pathway. We postulated that CL oxidation mobilizes not only cyt c but also CL itself in the form of hydroperoxide (CLOOH) species. Relatively hydrophilic CLOOHs could assist in apoptotic signaling by translocating to the outer membrane (OM), thus promoting recruitment of the pro-apoptotic proteins truncated Bid (tBid) and Bax for generation of cyt c-traversable pores. Initial testing of these possibilities showed that CLOOH-containing liposomes were permeabilized more readily by tBid plus Ca(2+) than CL-containing counterparts. Moreover, CLOOH translocated more rapidly from IM-mimetic to OM-mimetic liposomes than CL and permitted more extensive OM permeabilization. We found that tBid bound more avidly to CLOOH-containing membranes than to CL counterparts, and binding increased with increasing CLOOH content. Permeabilization of CLOOH-containing liposomes in the presence of tBid could be triggered by monomeric Bax, consistent with tBid/Bax cooperation in pore formation. Using CL-null mitochondria from a yeast mutant, we found that tBid binding and cyt c release were dramatically enhanced by transfer acquisition of CLOOH. Additionally, we observed a pre-apoptotic IM-to-OM transfer of oxidized CL in cardiomyocytes treated with the Complex III blocker, antimycin A. These findings provide new mechanistic insights into the role of CL oxidation in the intrinsic pathway of oxidative apoptosis.
Figures


References
-
- Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. (1996) Cell 86, 147–157 - PubMed
-
- Newmeyer D. D., Ferguson-Miller S. (2003) Cell 112, 481–490 - PubMed
-
- Garrido C., Galluzzi L., Brunet M., Puig P. E., Didelot C., Kroemer G. (2006) Cell Death Differ. 13, 1423–1433 - PubMed
-
- Shidoji Y., Hayashi K., Komura S., Ohishi N., Yagi K. (1999) Biochem. Biophys. Res. Commun. 264, 343–347 - PubMed
-
- Petrosillo G., Ruggiero F. M., Pistolese M., Paradies G. (2001) FEBS Lett. 509, 435–438 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

