Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone
- PMID: 21642436
- PMCID: PMC3143656
- DOI: 10.1074/jbc.M111.249532
Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone
Abstract
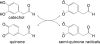
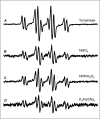
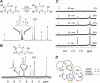
The oxidation and toxicity of dopamine is believed to contribute to the selective neurodegeneration associated with Parkinson disease. The formation of reactive radicals and quinones greatly contributes to dopaminergic toxicity through a variety of mechanisms. The physiological metabolism of dopamine to 3,4-dihydroxyphenylacetaldehyde (DOPAL) via monoamine oxidase significantly increases its toxicity. To more adequately explain this enhanced toxicity, we hypothesized that DOPAL is capable of forming radical and quinone species upon oxidation. Here, two unique oxidation products of DOPAL are identified. Several different oxidation methods gave rise to a transient DOPAL semiquinone radical, which was characterized by electron paramagnetic resonance spectroscopy. NMR identified the second oxidation product of DOPAL as the ortho-quinone. Also, carbonyl hydration of DOPAL in aqueous media was evident via NMR. Interestingly, the DOPAL quinone exists exclusively in the hydrated form. Furthermore, the enzymatic and chemical oxidation of DOPAL greatly enhance protein cross-linking, whereas auto-oxidation results in the production of superoxide. Also, DOPAL was shown to be susceptible to oxidation by cyclooxygenase-2 (COX-2). The involvement of this physiologically relevant enzyme in both oxidative stress and Parkinson disease underscores the potential importance of DOPAL in the pathogenesis of this condition.
Figures
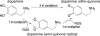





References
-
- Davie C. A. (2008) Br. Med. Bull. 86, 109–127 - PubMed
-
- Fornstedt B., Brun A., Rosengren E., Carlsson A. (1989) J. Neural Transm. Park. Dis. Dement. Sect. 1, 279–295 - PubMed
-
- Collins M. A., Neafsey E. J. (2002) Neurotoxicol. Teratol. 24, 571–577 - PubMed
-
- Terland O., Almas B., Flatmark T., Andersson K. K., Sorlie M. (2006) Free Radic. Biol. Med. 41, 1266–1271 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

