Rediscovering sperm ion channels with the patch-clamp technique
- PMID: 21642646
- PMCID: PMC3136206
- DOI: 10.1093/molehr/gar044
Rediscovering sperm ion channels with the patch-clamp technique
Abstract
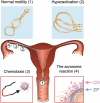
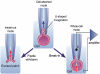
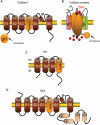
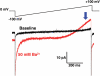
Upon ejaculation, mammalian spermatozoa have to undergo a sequence of physiological transformations within the female reproductive tract that will allow them to reach and fertilize the egg. These include initiation of motility, hyperactivation of motility and perhaps chemotaxis toward the egg, and culminate in the acrosome reaction that permits sperm to penetrate the protective vestments of the egg. These physiological responses are triggered through the activation of sperm ion channels that cause elevations of sperm intracellular pH and Ca(2+) in response to certain cues within the female reproductive tract. Despite their key role in sperm physiology and their absolute requirement for the process of fertilization, sperm ion channels remain poorly understood due to the extreme difficulty in application of the patch-clamp technique to spermatozoa. This review covers the topic of sperm ion channels in the following order: first, we discuss how the intracellular Ca(2+) and pH signaling mediated by sperm ion channels controls sperm behavior during the process of fertilization. Then, we briefly cover the history of the methodology to study sperm ion channels, which culminated in the recent development of a reproducible whole-cell patch-clamp technique for mouse and human cells. We further discuss the main approaches used to patch-clamp mature mouse and human spermatozoa. Finally, we focus on the newly discovered sperm ion channels CatSper, KSper (Slo3) and HSper (H(v)1), identified by the sperm patch-clamp technique. We conclude that the patch-clamp technique has markedly improved and shifted our understanding of the sperm ion channels, in addition to revealing significant species-specific differences in these channels. This method is critical for identification of the molecular mechanisms that control sperm behavior within the female reproductive tract and make fertilization possible.
Figures


References
-
- Acott TS, Carr DW. Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor . Biol Reprod. 1984;30:926–935. - PubMed
-
- Aitken RJ, Irvine S, Kelly RW. Significance of intracellular calcium and cyclic adenosine 3',5′-monophosphate in the mechanisms by which prostaglandins influence human sperm function. J Reprod Fertil. 1986;77:451–462. doi:10.1530/jrf.0.0770451. - DOI - PubMed
-
- Alabi AA, Bahamonde MI, Jung HJ, Kim JI, Swartz KJ. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450:370–375. doi:10.1038/nature06266. - DOI - PMC - PubMed
-
- Arnoult C, Cardullo RA, Lemos JR, Florman HM. Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc Natl Acad Sci USA. 1996;93:13004–13009. doi:10.1073/pnas.93.23.13004. - DOI - PMC - PubMed
-
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

