Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5
- PMID: 21642957
- PMCID: PMC3155310
- DOI: 10.1038/emboj.2011.179
Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5
Abstract
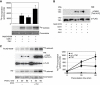
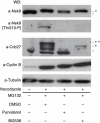
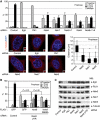
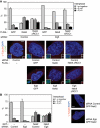
The NIMA-family kinases Nek9/Nercc1, Nek6 and Nek7 form a signalling module required for mitotic spindle assembly. Nek9, the upstream kinase, is activated during prophase at centrosomes although the details of this have remained elusive. We now identify Plk1 as Nek9 direct activator and propose a two-step activation mechanism that involves Nek9 sequential phosphorylation by CDK1 and Plk1. Furthermore, we show that Plk1 controls prophase centrosome separation through the activation of Nek9 and ultimately the phosphorylation of the mitotic kinesin Eg5 at Ser1033, a Nek6/7 site that together with the CDK1 site Thr926 we establish contributes to the accumulation of Eg5 at centrosomes and is necessary for subsequent centrosome separation and timely mitosis. Our results provide a basis to understand signalling downstream of Plk1 and shed light on the role of Eg5, Plk1 and the NIMA-family kinases in the control of centrosome separation and normal mitotic progression.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

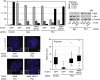

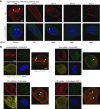

References
-
- Archambault V, Glover DM (2009) Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 10: 265–275 - PubMed
-
- Belham C, Roig J, Caldwell JA, Aoyama Y, Kemp BE, Comb M, Avruch J (2003) A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J Biol Chem 278: 34897–34909 - PubMed
-
- Blangy A, Arnaud L, Nigg EA (1997) Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150. J Biol Chem 272: 19418–19424 - PubMed
-
- Blangy A, Lane HA, d’Hérin P, Harper M, Kress M, Nigg EA (1995) Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83: 1159–1169 - PubMed
-
- Elia AE, Cantley LC, Yaffe MB (2003) Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 299: 1228–1231 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

