Metal Complexes for DNA-Mediated Charge Transport
- PMID: 21643528
- PMCID: PMC3105778
- DOI: 10.1016/j.ccr.2010.09.002
Metal Complexes for DNA-Mediated Charge Transport
Abstract
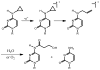
In all organisms, oxidation threatens the integrity of the genome. DNA-mediated charge transport (CT) may play an important role in the generation and repair of this oxidative damage. In studies involving long-range CT from intercalating Ru and Rh complexes to 5'-GG-3' sites, we have examined the efficiency of CT as a function of distance, temperature, and the electronic coupling of metal oxidants bound to the base stack. Most striking is the shallow distance dependence and the sensitivity of DNA CT to how the metal complexes are stacked in the helix. Experiments with cyclopropylamine-modified bases have revealed that charge occupation occurs at all sites along the bridge. Using Ir complexes, we have seen that the process of DNA-mediated reduction is very similar to that of DNA-mediated oxidation. Studies involving metalloproteins have, furthermore, shown that their redox activity is DNA-dependent and can be DNA-mediated. Long range DNA-mediated CT can facilitate the oxidation of DNA-bound base excision repair proteins to initiate a redox-active search for DNA lesions. DNA CT can also activate the transcription factor SoxR, triggering a cellular response to oxidative stress. Indeed, these studies show that within the cell, redox-active proteins may utilize the same chemistry as that of synthetic metal complexes in vitro, and these proteins may harness DNA-mediated CT to reduce damage to the genome and regulate cellular processes.
Figures

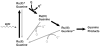
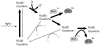
References
-
- van Loon B, Markkanen E, Hübscher U. DNA Repair. 2010;9:604. - PubMed
-
- Møller P, Folkmann JK, Forchhammer L, Bräuner EV, Danielsen PH, Risom L, Loft S. Cancer Lett. 2008;266:84. - PubMed
-
- Cooke MS, Evans MD, Dizdaroglu M. J Lunec FASEB J. 2003;17:1195. - PubMed
-
- Nishikawa M. Cancer Lett. 2008;266:53. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
