Total synthesis of pinnatoxins A and G and revision of the mode of action of pinnatoxin A
- PMID: 21644584
- PMCID: PMC3365589
- DOI: 10.1021/ja201254c
Total synthesis of pinnatoxins A and G and revision of the mode of action of pinnatoxin A
Abstract
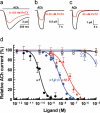
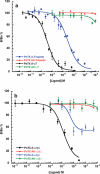
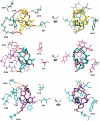
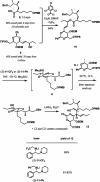
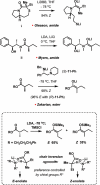
Pinnatoxins belong to an emerging class of potent marine toxins of the cyclic imine group. Detailed studies of their biological effects have been impeded by unavailability of the complex natural product from natural sources. This work describes the development of a robust, scalable synthetic sequence relying on a convergent strategy that delivered a sufficient amount of the toxin for detailed biological studies and its commercialization for use by other research groups and regulatory agencies. A central transformation in the synthesis is the highly diastereoselective Ireland-Claisen rearrangement of a complex α,α-disubstituted allylic ester based on a unique mode for stereoselective enolization through a chirality match between the substrate and the lithium amide base. With synthetic pinnatoxin A, a detailed study has been performed that provides conclusive evidence for its mode of action as a potent inhibitor of nicotinic acetylcholine receptors selective for the human neuronal α7 subtype. The comprehensive electrophysiological, biochemical, and computational studies support the view that the spiroimine subunit of pinnatoxins is critical for blocking nicotinic acetylcholine receptor subtypes, as evidenced by analyzing the effect of a synthetic analogue of pinnatoxin A containing an open form of the imine ring. Our studies have paved the way for the production of certified standards to be used for mass-spectrometric determination of these toxins in marine matrices and for the development of tests to detect these toxins in contaminated shellfish.
Figures

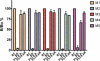
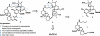
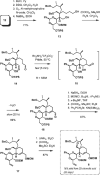

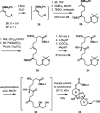
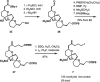

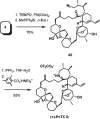

References
-
- Cembella A, Krock B. In: Seafood and Freshwater Toxins. 2nd ed. Botana LM, editor. CRC Press; Boca Raton, FL: 2008. pp. 561–580.
-
- Gueret SM, Brimble MA. Nat. Prod. Rep. 2010;27:1350–1366. - PubMed
-
- Munday R. In: Seafood and Freshwater Toxins. 2nd ed. Botana LM, editor. CRC Press; Boca Raton, FL: 2008. pp. 581–594.
-
- Aune T. In: Seafood and Freshwater Toxins. 2nd ed. Botana LM, editor. CRC Press; Boca Raton, FL: 2008. pp. 3–20.
-
- Hu T, Burton IW, Cembella AD, Curtis JM, Quilliam MA, Walter JA, Wright JLC. J. Nat. Prod. 2001;64:308–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

