Niche-specific contribution to streptococcal virulence of a MalR-regulated carbohydrate binding protein
- PMID: 21645132
- PMCID: PMC3147245
- DOI: 10.1111/j.1365-2958.2011.07708.x
Niche-specific contribution to streptococcal virulence of a MalR-regulated carbohydrate binding protein
Abstract
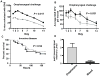
Low G+C Gram-positive bacteria typically contain multiple LacI/GalR regulator family members, which often have highly similar amino-terminal DNA binding domains, suggesting significant overlap in target DNA sequences. The LacI/GalR family regulator catabolite control protein A (CcpA) is a global regulator of the Group A Streptococcus (GAS) transcriptome and contributes to GAS virulence in diverse infection sites. Herein, we studied the role of the maltose repressor (MalR), another LacI/GalR family member, in GAS global gene expression and virulence. MalR inactivation reduced GAS colonization of the mouse oropharynx but did not detrimentally affect invasive infection. The MalR transcriptome was limited to only 25 genes, and a highly conserved MalR DNA-binding sequence was identified. Variation of the MalR binding sequence significantly reduced MalR binding in vitro. In contrast, CcpA bound to the same DNA sequences as MalR but tolerated variation in the promoter sequences with minimal change in binding affinity. Inactivation of pulA, a MalR regulated gene which encodes a cell surface carbohydrate binding protein, significantly reduced GAS human epithelial cell adhesion and mouse oropharyngeal colonization but did not affect GAS invasive disease. These data delineate a molecular mechanism by which hierarchical regulation of carbon source utilization influences bacterial pathogenesis in a site-specific fashion.
© 2011 Blackwell Publishing Ltd.
Figures

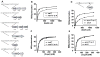



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

