Cholesterol esters as growth regulators of lymphocytic leukaemia cells
- PMID: 21645151
- PMCID: PMC6496738
- DOI: 10.1111/j.1365-2184.2011.00758.x
Cholesterol esters as growth regulators of lymphocytic leukaemia cells
Abstract
Objective: Alterations in plasma lipid profile and in intracellular cholesterol homoeostasis have been described in various malignancies; however, significance of these alterations, if any, in cancer biology is not clear. The aim of the present study was to investigate a possible correlation between alterations in cholesterol metabolism and expansion of leukaemia cell numbers.
Materials and methods: Lipid profiles in plasma and in primary leukaemia cells isolated from patients with acute or chronic lymphocytic leukaemia (ALL and CLL) were studied.
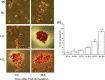
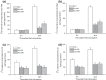
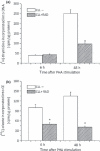
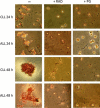
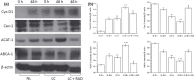
Results and conclusions: Decreased levels of HDL-C were observed in plasma of leukaemic patients, levels of total cholesterol, LDL-C, triglycerides and phospholipids were unchanged or only slightly increased. As compared to normal lymphocytes, freshly isolated leukaemic cells showed increased levels of cholesterol esters and reduction in free cholesterol. Growth stimulation of ALL and CLL cells with phytohemagglutinin led to further increase in levels of cholesterol esters. Conversely, treatment with an inhibitor of cell proliferation such as the mTOR inhibitor, RAD, caused decline in population growth rate of leukaemia cells, which was preceded by sharp reduction in rate of cholesterol esterification. On the other hand, exposure of leukaemic cells to two inhibitors of cholesterol esterification, progesterone and SaH 58-035, caused 60% reduction in their proliferation rate. In addition to demonstrating tight correlation between cell number expansion and cholesterol esterification in leukaemic cells, these results suggest that pathways that control cholesterol esterification might represent a promising targets for novel anticancer strategies.
© 2011 Blackwell Publishing Ltd.
Figures



References
-
- Roffo AH (1916) Montevideo: An. Facult. Med..
-
- Roffo AH (1933) Heliotropism of cholesterol in relation to skin cancer. Am. J. Cancer. 17, 42–57.
-
- Roffo AH, Pilar F (1930) Bol. Inst. Med.Eexp. Estud. y Trat. Cancer 7, 22
-
- Pani A, Dessi S (2003) Cell growth and cholesterol ester. Springer Publication 1–11, 1–150.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

