Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation
- PMID: 21645255
- PMCID: PMC3140222
- DOI: 10.1111/j.1600-6143.2011.03566.x
Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation
Abstract
We recently reported long-term organ allograft survival without ongoing immunosuppression in four of five patients receiving combined kidney and bone marrow transplantation from haploidentical donors following nonmyeloablative conditioning. In vitro assays up to 18 months revealed donor-specific unresponsiveness. We now demonstrate that T cell recovery is gradual and is characterized by memory-type cell predominance and an increased proportion of CD4⁺ CD25⁺ CD127⁻ FOXP3⁺ Treg during the lymphopenic period. Complete donor-specific unresponsiveness in proliferative and cytotoxic assays, and in limiting dilution analyses of IL-2-producing and cytotoxic cells, developed and persisted for the 3-year follow-up in all patients, and extended to donor renal tubular epithelial cells. Assays in two of four patients were consistent with a role for a suppressive tolerance mechanism at 6 months to 1 year, but later (≥ 18 months) studies on all four patients provided no evidence for a suppressive mechanism. Our studies demonstrate, for the first time, long-term, systemic donor-specific unresponsiveness in patients with HLA-mismatched allograft tolerance. While regulatory cells may play an early role, long-term tolerance appears to be maintained by a deletion or anergy mechanism.
©2011 The Authors Journal compilation©2011 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest as described by the American Journal of Transplantation
Figures
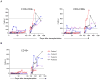



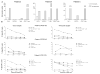

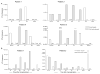
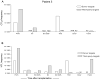
References
-
- Kawai T, Cosimi AB, COLVIN RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomologous monkeys. Transplantation. 1995;59:256–62. - PubMed
-
- Sharabi Y, Sachs DH, Sykes M. T cell subsets resisting induction of mixed chimerism across various histocompatibility barriers. In: Gergely J, Benczur M, Falus A, Fust Gy, Medgyesi G, Petranyi Gy, et al., editors. Progress in Immunology VIII; Proceedings of the Eighth International Congress of Immunology; Budapest. 1992; Heidelberg: Springer-Verlag; 1992. pp. 801–5.
-
- Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma Responses and Tolerance Following Combined Kidney and Nonmyeloablative Marrow Transplantation: In Vivo and In Vitro Analyses. Am J Transplant. 2006 Jun 22;6(9):2121–33. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

